mineral nutrition of pigs
New perspectives on mineral nutrition of pigs
Introduction
All animal tissues contain mineral elements in variable amounts and minerals are needed for efficient pig production. Most of the advances in understanding the nutritional significance of minerals were made during the second quarter of the 20th century and the majority of the studies focused on requirements to avoid deficiencies in the different phases of production. Based on available information the requirements of pigs for calcium (Ca) and phosphorus (P) are reasonably well known, but the information on electrolyte requirements, especially for piglets, is scarce. Moreover, the available data on trace mineral needs and related effects on aspects of animal production such as immune function, resistance to disease, growth promotion, and carcass composition are limited. In the last two decades the negative effects of excess amounts of certain minerals on the absorption of others and on the contamination of the environment have became important. In this respect, the negative impact of P on the environment is the most studied, but contamination by copper (Cu), zinc (Zn), sodium (Na), and other minerals is also important.
Inappropriate application of pig manure to cropland has negative effects on water and soils. Decreasing the mineral content of manure through nutritional changes of the diet is, compared to other alternatives, a powerful, cost-effective approach to reduce environmental contamination from swine farms. Reducing 'safety' margins, phase feeding, selecting mineral sources according to bioavailability, and the use of phytases will reduce mineral contamination and also cost of production, helping farmers to meet environmental regulations. Success will depend on accurate estimates of requirements for each type of production. Advanced methods to study the digestibility of minerals for pigs will help to implement these strategies. The objectives of this paper are to review new perspectives on the use of minerals in pig diets and their impact on pig health and production and maintenance of the environment.
Macrominerals
Macrominerals are those elements whose requirements are expressed as a percentage of the diet or in g/head/ day. Calcium, P, magnesium (Mg), sodium (Na), chlorine (Cl), potassium (K), and sulfur (S) are the main macrominerals considered in animal feeding, but only P, Ca, and Na are commonly added to pig diets.
Sometimes Mg is also added, although the scientific evidence justifying its addition in pig diets is scant.
CALCIUM
Calcium is the most abundant mineral in the body, and 99% is found in the skeleton. Therefore, the main quantitative function of Ca in the organism is to provide support, allow movement, and protect key organs from injury. The requirement for Ca depends on the physiological state of the pig, availability of the source used, interaction with other minerals, and the amount of vitamin D provided. For example, Ca requirements increase in the late stages of gestation and in lactating sows. Also, the adequacy of a particular dietary concentration of Ca varies with the vitamin D status of the animal. A deficiency in vitamin D will show similar symptoms as a deficiency in Ca. Finally, outdoor pigs, which are allowed to exercise and have direct access to sunlight, will require less Ca for bone strength and leg soundness than pigs reared in enclosed facilities.
Numerous feeding trials have been conducted to define the Ca requirements of pigs. The ARC (1981) tabulated the results of 56 trials published between 1964 and 1976 and concluded that recommendations for Ca inclusion had increased substantially in the last 10 years (by up to 50%). However, these high Ca intake recommendations are probably not needed. Part of the problem was that ARC (1981) assumed that the efficiency of absorption declined from 67% to 47% as pigs grew from 25 to 90 kg live weight. In addition, the method used for calculations did not take into account the variability in Ca absorption among sources (Underwood and Suttle, 1999). It is known that the digestibility of Ca is greater for ingredients of animal or mineral origin than for ingredients from vegetable sources. Also, Ca from calcium carbonate is more available than that from monocalcium phosphate. Therefore, total Ca requirements will be relatively smaller for piglets fed diets based on animal ingredients than for finishing pigs, which are fed diets based on vegetable ingredients. In fact, Mahan (1982) indicated that the total dietary Ca concentration necessary for weanling pigs to attain maximum bone ash for 7 to 20 kg body weight was 0.8%, whereas only 0.7% was needed to maximise performance. The experimental diets in this trial contained 0.68% total P and 0.35% available P. The decrease observed in the last decade in Ca content of pig feeds is due in part to better knowledge of pig needs and to the use of phytases (Kemme et al., 1999). In fact, the Ca level of piglet diets in Spain has decreased from 1.1% to 0.65% in the last 15 years without any negative effect on performance.
The amount of Ca (and P) in the diet plays an important role in sow productivity and welfare.
However, there is no agreement among authors on the optimum level of Ca in sow diets. Kornegay et al. (1973) reported that increased levels of Ca and P seemed to reduce the number of sows that were crippled because of broken femurs, failed to breed, or that died for unspecified reasons. Furthermore, Nimmo et al. (1981) found a greater incidence of leg problems in gilts fed low Ca (0.65%) and P (0.50%) diets during both growth and gestation than in gilts fed higher levels. In contrast, Kornegay et al. (1985) did not observe any increase in longevity of sows fed high levels of Ca and P during growth and development. Giesemann et al. (1998) indicated that young sows (first and second parity) absorbed and retained more Ca and P during gestation than old sows (fifth and sixth parity). However, during lactation, retention of Ca and P was limited in young sows, which were also more vulnerable to bone fracture than old sows. Therefore, more research is needed on ways to maximise bone weight of young sows and to minimise bone losses during lactation.
Calcium sources are not expensive, and in practice excess is probably more of a problem than deficiency. In fact, we have analysed 910 pig feeds in the past five years and found that in 60% of the samples the level of Ca analysed in the laboratory exceeded the expected value by more than 10%. However, only in 15% of the samples were the values analysed below those expected.
Three reasons help to explain these observations: 1) Ca sources are cheap and in consequence less attention is paid to an excess, 2) equipment in feed mills to dose Ca is not adequate, and unnecessary extra amounts of limestone flow into the mixer, and 3) calcium carbonate is often added to ingredients as an inert carrier (as in premixes) or to facilitate ingredient flow through the transport system (as in some imported cereal by-products and soybean meal) but it is not accounted for in formulation.
In some instances, an excess of Ca should be avoided.
For example, Ca sources increase the buffering capacity of piglet feeds, which in turn reduces the acidity of the stomach contents and might decrease the digestibility of proteins of vegetable origin and increase the incidence of diarrhoea. Also, an excess of Ca in the diet will reduce phytase activity and the absorption of P and other ions such as Zn and Cu. Eeckhout et al. (1995) have observed that the availability of Ca from calcium carbonate (CaCO3) is much higher than from calcium monophosphate (CaHPO4). Therefore, when CaCO3 is included in piglet diets there is a rapid rise in the ratio of available Ca:available P, which reduces P absorption. In countries in which excess P in the diet is penalized, the dietary level of P is close to the minimum requirement with little or no safety margin. In this situation an excess of Ca might jeopardize the P status and reduce pig performance, because excess Ca is more critical when for economical or environmental reasons the P level in the diet is very low. Similarly, a given concentration of Zn in the diet can be inadequate when Ca intake is high. Finally, an excess of Ca in the diet as calcium carbonate or calcium chloride may reduce palatability, and therefore feed intake of piglets.
Therefore, in modern pig production it is important to feed to minimum Ca requirements in order to reduce buffering capacity of the diet and to make the most efficient use of phytate P.
PHOSPHORUS
Phosphorus is the second most abundant mineral in the animal body and about 80% is found in the bones and teeth. Quantitatively the most important function of P is the formation of the organic bone matrix as well as the mineralisation of that matrix. However, P is also essential in cell growth and differentiation; and as a component of phospholipids P contributes to cell membrane fluidity and integrity. In addition, ionic P helps maintain acid-base balance and plays a vital role in many metabolic functions, including energy utilisation and transfer, synthesis of proteins, and function of the Na+/K+ pump. In addition to bone abnormalities, a deficiency in P causes poor appetite and feed utilization, reproductive disturbances, and 'pica' or aberrant behaviour (Underwood and Suttle, 1999).
The NRC (1998) has established an available P requirement of 0.4% for piglets from 5 to 10 kg, declining to 0.19% for 50 to 80 kg pigs, and to 0.15% for pigs of 80 to 120 kg body weight. In general, companies supplying breeding stock recommend higher levels of available P than NRC (1998) and the tendency of the industry is to match, if possible, genetic guidelines.
Furthermore, breeding companies recommend up to 0.5% available P for starter feeds (6 to 20 kg), 0.45% for grower feeds (20 to 60 kg), and 0.42% for finisher feeds (60 to 110 kg) (Hypor, 2000; PIC, 2002).
However, during the later stages of production, when a significant amount of feed is consumed, there is little need for supplemental P in a typical corn-soybean meal diet (Mavromichalis et al., 1999). Field observations indicate that finishing pigs perform well even in the absence of any mineral source of P for the last 30 days of the fattening period (McGlone, 2000). However, research has shown that, in relative terms, more P is needed to maximise bone strength than to maximise body weight or feed efficiency. Therefore, very low levels of P in the diet reduce bone ash and breaking strength and should be avoided in pigs reared for breeding purposes.
In pigs the absorption coefficients of P sources range from 50 to 85% and are affected by many factors including physiological state, phytase addition, and Ca content of the diet. Table 1 shows the P content and bioavailability (availability or digestibility) of selected ingredients according to Iowa State University (1996) and FEDNA (2003) of key ingredients used in pig diets.
The methods of P evaluation based on available P, as recommended in the US, tended to underestimate mineral sources of P with respect to vegetable sources.
Also, the data from Iowa State University (1996) indicate that the availability (relative values as compared to monosodium phosphate) of all mineral sources are the same. However, methods based on digestible P, as those recommended in many EU-25 countries, establish wide differences in digestibility (absolute values) among mineral sources. In fact, FEDNA (2003) estimates a digestibility coefficient of 92% for monocalcium phosphate but only 76% for dicalcium phosphate.
Similar data have been published recently by INRAAFZ (2002) indicating that the relative biological value of P of hydrated dicalcium phosphate is much lower than that of monocalcium phosphate (77 vs 92%) (Table 2). However, the variability in digestible values among processors producing the same mineral source is high and therefore, average values are only 'good' estimates of the real value.
Table 1. Bioavailability of sources of phosphorus for pigs (%).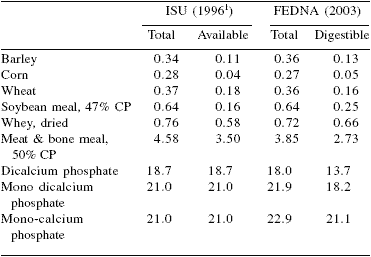
1Iowa State University
Table 2.Relative biological values of mineral sources of phosphorus for pigs.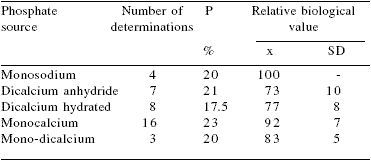
INRA-AFZ, 2002
The P content of pig diets has been reduced considerably in the EU-25 in the last 15 years. The high cost of P sources, the use of digestible rather than available P in formulation, the inclusion of phytase, the reduction in dietary Ca, and the environmental issue are responsible for the reduction. However, still more work is needed to further reduce the P content of commercial pig diets (Ferket et al., 2002).
MAGNESIUM
Magnesium has many physiological functions and is the second most plentiful cation (after potassium) in intracellular fluids. Magnesium is involved in more than 300 enzymatic metabolic reactions and is an essential activator of many enzymes involved in the Krebs cycle.
A deficiency of Mg affects growth, immunity, and muscle contraction. Although Mg is an extremely important nutrient, pig requirements are very low. Since natural ingredients such as cereals and oilseed meals are good sources of Mg, a deficiency is unlikely in practical production systems. Recently, different researchers (D'Souza et al., 1998, 1999; Hamilton et al., 2002; Lahucky et al., 2004) have found that Mg salts are effective in reducing the incidence of pale, soft, exudative (PSE) pork and improved meat colour compared to controls. Hamilton et al. (2003) reported that just 1.6 g Mg/pig/day administered through feed as sulphate, propionate or proteinate for one day prior to slaughter was effective in improving pork colour and water-holding capacity. However, other authors (Kuhn et al., 1981) have not found any benefit on pork quality of including extra amounts of Mg in the diet.
SODIUM AND CHLORINE
High levels of Na (and salt) in the diet increase water intake, promote salt accumulation in manure pits, and increase the salinity of soils treated with these wastes.
Therefore, excessive amounts of added salt in pig diets should be avoided on behalf of the environment.
Research has shown that a minimum amount of 0.20% salt is required for fattening pigs fed a corn-soybean meal diet from 18 to 100 kg (Ib Hagsten et al., 1976).
Therefore, the dietary Na and chlorine (Cl) requirements of growing-finishing pigs are no greater than 0.10% and 0.08% of the diet, respectively (NRC, 1998).
However, field information indicates that increasing the level of salt from 0.20 to 0.50% or more reduced the incidence of cannibalism, and tended to improve feed intake under hot weather conditions. Additionally, swine can tolerate high dietary levels of NaCl provided they have access to non-saline drinking water. Also, low salt intake by sows (0.25 vs 0.50% added salt in the diet) tended to reduce birth weight and litter size at birth and weaning (Cromwell et al., 1989). Reduced levels of salt in the diet also increase the interval from weaning to oestrus (Seynaeve et al., 1996). Based on the Na content of sow's milk (0.035%) and feed intake, the dietary Na requirement should be about 0.05 percentage units greater during lactation than during gestation. Until recently, the recommended levels of Na and Cl of piglet feeds were low, and in practice many nutritionists limited the amount of these elements in the formula, assuming that an excess of Na and Cl was responsible for the high incidence of scours observed in the field. It was assumed that animal products, especially fish meal and dried whey, were good sources of minerals and that extra amounts of Na or Cl were not needed. However, new experiments demonstrated that this assumption was not necessarily valid and the NRC (1998) increased by 100%, and 250%, respectively, the recommended level of Na and Cl with respect to former recommendations (NRC, 1988). More recently, Mahan et al. (1999) have observed that levels of Na and Cl in the diet higher than those recommended by NRC (1998) improved piglet performance further (Table 3). These authors recommend a dietary minimum of 0.38% total Cl and indicated that a level of Na over 0.28% might benefit piglet growth over current NRC (1998) recommendations (Table 4).
Table 3.Effect of added dietary sodium and chorine on performance of 3-week-old weanling pigs1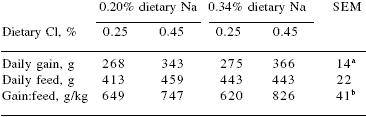
Mahan et al., 1999
129 to 36 days of age
aChlorine response (P<0.01)
bChlorine response (P<0.05)
Table 4.Requirements for sodium and chlorine of piglets (% of diet1).
15 to 10 kg body weight
2Estimated from published data
Numerous research reports have focused on the effects of dietary electrolyte balance (dEB) on growth performance of both pigs and poultry. However, electrolyte balance of the diet is not easy to define and its effect on pig performance is not well established (Patience and Chaplin, 1997). In monogastrics, dEB is defined as N+ + K+ - Cl- in meq/kg of diet. Practical swine grower diets based on corn and soybean meal possess a dEB of about 150 to 175 meq/kg. It is believed that an excess of Cl over Na and K produces acidosis, which might create metabolic disturbances and reduce amino acid digestibility and feed intake. The reason is not fully understood, but Dersjant-Li et al. (2002) have found that reducing the dEB lowered both arterial and portal blood haemoglobin and oxygen content, which may partially explain the reduced performance of pigs fed these type of diets. Also, Yen et al. (1981) indicated that the ingestion by the pig of a diet containing 4% calcium chloride (CaCl2) increased plasma Cl concentration, producing a metabolic acidosis thereby appearing to suppress appetite. In pigs, a dEB of 175 meq/kg or more is recommended (Patience et al., 1987; Haydon and West, 1990), but the research to support this recommendation is scarce. In a recent report, De Rouchey et al. (2003) evaluated the effects of dEB on sow performance. Sows (average parity 2.2) were offered similar diets ranging in dEB from 0 to 500 meq/kg. Increasing dEB increased blood and urine pH and urinary microbial load. Feed intake, water usage, litter growth, and backfat of sows were not affected by diet. However, piglet survival and the number of pigs weaned increased with decreasing dEB value.
Practical concentrations of macrominerals in pig diets vary widely depending on factors that are difficult to control. Of these factors the most important are the safety margin required by the nutritionist and the pressure imposed by local legislation. The recommended levels of Ca, P, Mg, Na, Cl, and K for piglets, growingfattening pigs, gestating, and lactating sows according to selected sources are shown in Tables 5, 6, 7, and 8, respectively.
Table 5.Macromineral requirements of piglets (% of diet).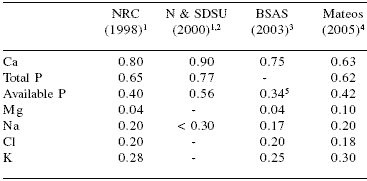
15 to 10 kg BW
2Nebraska and South Dakota State University
310 to 30 kg BW
4Practical levels of use (unpublished data)
5Digestible P
Table 6.Macromineral requirements of growing/fattening pigs (% of diet).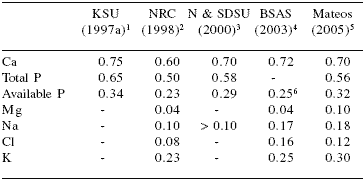
1Kansas State University (30 to 60 kg BW)
220 to 50 kg BW. No changes from NRC (1988)
3Nebraska and South Dakota State University (20 to 36 kg BW)
422 to 36 kg BW
5 Practical levels of use from 20 to 60 kg BW (unpublished data)
6Digestible P
Table 7.Macromineral requirements of gestating sows (% of diet).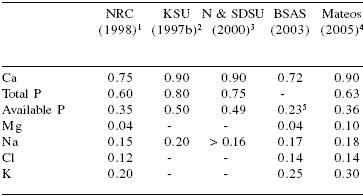
1No changes from NRC (1988)
2Kansas State University
3Nebraska and South Dakota State University
4Practical levels of use (unpublished data)
5Digestible P
Table 8. Macromineral requirements of lactating sows (% of diet).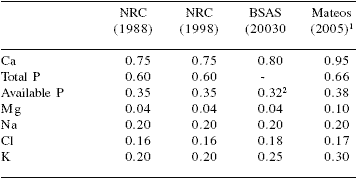
1Practical levels of use (unpublished data)
2Digestible P
Trace minerals
Trace minerals are needed in small amounts in the diet and the requirements are expressed as mg per day or as mg/kg of diet. Iron (Fe), copper (Cu), zinc (Zn), manganese (Mn), selenium (Se), and iodine (I) are routinely included in trace mineral premixes for swine.
Many premixes also include cobalt (Co), chromium (Cr), and molybdenum (Mo) although their need in pig feeds is less evident. A comparison between the composition of commercial trace mineral premixes in Spain (Mateos et al., 2005) and the requirements recommended by NRC (1998) and BSAS (2003) for piglets, growing/ fattening pigs, and lactating sows are offered in Table 9. New regulations concerning the maximum levels of trace minerals allowed in pig diets in the European Union (EU-25) have imposed pressure on nutritionists to minimise trace mineral inclusion (Doce, 2003; 2004).
Also, because of safety reasons and increased control of contaminants, the cost of mineral salts used as sources of trace minerals in premixes has increased and therefore, extra attention must be paid to formulate mineral premixes at a competitive price. The maximum levels of trace minerals allowed in pig diets in the EU-25 are shown in Table 10. A review of pig requirements and symptoms of deficiencies of trace minerals has been presented elsewhere (Mateos et al., 2004; 2005). In this work we will only emphasise the most important points related to trace mineral use in practical pig diets.
Table 9.Trace mineral recommendations (mg/kg) of diets for pigs.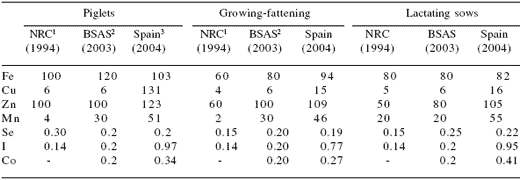
15 to 10 kg BW for piglets and 20 to 50 kg for growing/fattening
210 to 30 kg BW for piglets and 30 to 60 kg for growing/fattening
3Average of 35, 30, and 25 commercial premixes for piglets (6 to 12 kg) growing/fattening (12 to 103 kg), and lactating sows, respectively
(Mateos et al., 2005). Data for Cu for piglets include its use as a growth promoter.
Table 10.Maximal levels of trace minerals in mg/kg allowed in pig diets in the European Union-25.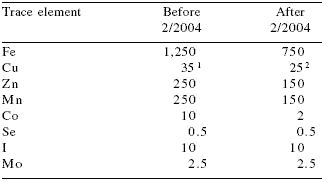
DOCE, 2003; 2004
1,2For piglets has passed from 175 ppm up to 16 weeks of age to
170 up to 12 weeks of age. Values depend on age and European
region considered (vulnerable zones)
IRON
Iron (Fe) is the most abundant trace element in the body where approximately 60% is present as haemoglobin. Iron is required for key biochemical functions such as DNA synthesis and oxygen (O2) transport and metabolism. The ease of oxidation and reduction of Fe makes it a unique trace element for many cellular redox reactions. Prolonged Fe deprivation is characterised by anaemia, loss of appetite, lethargy, increased respiration rate and higher mortality. In fact, Fe deficiency is the most common of the deficiency diseases in humans worldwide. However, added Fe supplementation has not improved pig performance in many trials, indicating that supplies are generally higher than requirements.
Plant materials used in pig feeds contain large amounts of Fe. Cereal grains contain 30 to 60 ppm Fe, leguminous seeds 60 to 100 ppm, and oilseed meals 200 to 400 ppm Fe. Finally, Ca and P are routinely added to pig diets as calcium carbonate and dicalciumor monocalcium phosphate, which have Fe content ranging from 600 to 800 ppm for the Ca sources and from 1,500 to 8,000 ppm for the P sources (NRC, 1998; Mateos et al., 2004). Therefore, the need for extra Fe supplementation of fattening pig diets is low. On the other hand, Fe crosses with difficulty both the placenta and the mammary gland barriers and in consequence, newborn piglets and sow milk have only low levels of this mineral. As a result, confined young pigs fed milk diets may need extra Fe supplementation.
A decreased resistance to infection with Fe deprivation has been described for rats and piglets. Iron-deficient pigs exhibit greater susceptibility to Escherichia coli endotoxins than healthy pigs (Osborne and Davis, 1968).
However, Fe is also essential for bacterial growth and excess Fe intake increases the amount available in the gastrointestinal tract and the occurrence and severity of bacterial infections. In fact, injection of Fe compounds reduces the survival rate of experimental animals infected with Clostridium perfringens or Salmonella typhimurium (Knight et al., 1983). Therefore, large doses of Fe could stimulate bacterial growth and be detrimental to pig health.
COPPER
Copper is essential for proper bone growth and development and is required for numerous enzymes involved in iron transport and metabolism, collagen formation, melanin production, immune function and integrity of the central nervous system. The concentrations of Cu needed to prevent physiological deficiencies are very low.
Although cereal grains and milk products are poor sources of Cu (2 to 10 ppm), oilseed meals are good sources (15 to 30 ppm). Thus, a Cu deficiency is unlikely in animals reared under intensive production systems when 5 to 10 ppm extra Cu is included in the diet. Yet, British studies conducted in the 1960s estimated that Cu sulphate at 250 ppm improved daily gains by 8% and feed conversion by 5.5% compared to controls (Barber et al., 1955; Braude, 1980). In consequence, most pig producers throughout the world include pharmacological levels of Cu in the diet (125 to 250 ppm) to enhance growth. It is estimated that the ban on use of copper sulphate (CuSO4) at pharmacological doses in diets fed growing/fattening pigs (16 weeks to slaughter) has impaired feed:gain ratio by approximately 40 g. The negative impact is more evident in farms with poor management and low hygiene status.
ZINC
Zinc is critically involved in cell replication and in the development of cartilage and bone, and a deficiency results in severe growth retardation, dermatitis (parakeratosis), and impaired reproduction both in the male and the female. Zinc participates in processes related to the production and regeneration of keratin and has a direct effect in the integrity of the udder lining and the protection of the mammary gland. Zinc requirements are high in piglets, but decrease rapidly with age. A Zn deficiency is more evident in high Ca, vegetable-based diets, because Ca may induce the formation of a Zn-Ca-phytate complex in the upper gastrointestinal tract. Therefore, the use of exogenous phytase and the reduction of the level of Ca in the diet might be beneficial for pig growth, especially with Zndeficient diets (Adeola et al., 1995; Ashida et al., 1999).
Feeding pharmacological levels of Zn to weanling pigs reduces scouring and increases performance (Shannon, 2005). Studies conducted in the 1990s demonstrated that 2,000 to 3,000 ppm Zn either as Zn oxide or Zn sulphate improved performance in the early stages of life (Hahn and Baker, 1993). These results have been confirmed by many other reports (Hill et al., 2000; 2001). However, other studies have not found a consistent response in pig growth to Zn supplementation (Schell and Kornegay, 1996; Augspurger et al., 2004).
In practical conditions, it is evident that supplying Zn oxide at a dose of 2,500 ppm Zn or higher for 14 days reduces the incidence of scours and improves piglet performance (Mateos et al., 2004). Therefore, Zn oxide is a potent and reliable growth-promoting agent in young pigs. Data from Hill et al. (2000) indicate that both Cu at 250 ppm and Zn at 3,000 ppm reduce scours and improve piglet growth. However, a combination of Cu at 250 ppm as CuSO4 and 3,000 ppm of Zn as Zn oxide does not improve piglet performance further (Table 11).
Table 11.Growth performance of weanling pigs fed high dietary concentrations of zinc and/or copper1.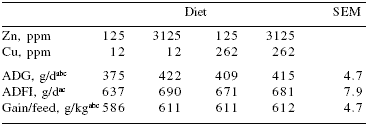
Hill et al., 2000
1From 22 to 50 days of age
aMain effect of Zn (P<0.01)
bMain effect of Cu (P<0.01)
cZn x Cu interaction (P<0.01)
MANGANESE
The nutritional importance of Mn for pigs has been an area of active investigation. Manganese is widely distributed in very low concentrations in body tissues and is necessary for enzyme activity, bone growth, lipid and carbohydrate metabolism, and reproductive function. The requirement for Mn in pigs is low and classical symptoms of deficiency are not observed in fattening pigs even in the absence of exogenous sources.
Pigs have been maintained from weaning to slaughter on semipurified diets containing as little as 1.5 ppm Mn without symptoms of deficiency (Liebholz et al., 1962). However, the Mn requirements for satisfactory reproduction are substantially higher than those needed for normal growth.
Apple et al. (2004) have observed that the inclusion of 320 to 350 ppm of an amino acid-Mn complex improved feed efficiency and meat quality in pigs. However, Kats et al. (1994) compared diets containing 24 or 88 ppm of Mn from either an inorganic or an organic source without finding any beneficial effect of the extra supplementation on growth performance, backfat thickness or longissimus muscle area of pigs. Therefore, more research is needed prior to recommending the practice of extra supplementation under commercial conditions.
SELENIUM
Selenium is a constituent of selenoproteins and plays both structural and enzymatic roles in pig nutrition. The history of Se as a nutrient in animal diets moved from prohibition as a toxic element to recognition of the need for supplementation in the diet. Originally, Se was recognised as a toxic mineral with carcinogenic properties and its utilisation in feeds was tightly controlled. In the pig, both organic and inorganic Se sources are toxic when fed at 20 ppm or more (acute poisoning) or at 5 to 10 ppm for prolonged periods (chronic toxicity) (Kim and Mahan, 2001a,b).
Paradoxically, it is now recognized that Se has powerful anti-cancer properties. In the EU-25 the current maximum level authorised for pigs is 0.5 ppm Se.
Beneficial effects of trace amounts of dietary Se were first observed in vitamin E-deficient rats. Selenium and vitamin E have mutual sparing effects for the prevention of certain nutritional diseases such as liver necrosis and exudative diathesis but the sparing effect is not found for other diseases (Surai, 2003). Selenium is a key element in antioxidant defence, and conditions associated with inflammation, such as occur at weaning, might be expected to be influenced by Se and vitamin E status. It seems that organic forms of Se, such as selenomethionine, are better suited to protect tissues from damage or to help in tissue repair than Se in the form of selenite (Surai, 2003). Mahan (2004) indicated that feeding organic Se from an enriched Se yeast (Sel-Plex®) to reproducing sows as compared with sodium selenite enhanced the Se status of both the sow and the progeny.
The organic Se form was transferred to colostrum and milk in much larger amounts than the inorganic Se form. However, both products resulted in equivalent plasma GSH-Px activities. Surai (2003) indicated that the transfer of organic Se from feed to the egg and embryonic tissues and from feed to the foetus, colostrum or milk was more efficient than the transfer of inorganic Se as selenite. The response is probably due to the metabolism and deposition of the Se as an integral part of body proteins.
Until recently it was considered that the main and almost unique role of Se in the organism was to be part of glutathione peroxidase (GSH-Px), an enzyme that maintained membrane integrity by neutralizing the negative effects of peroxides. New nutritional functions of Se include the production and regulation of the level of active thyroid hormone from thyroxin and the stabilisation of proteins required for sperm maturation and fertility (Rayman, 2002). For spermatogenic development and boar semen quality, the role of Se might be more important than the role of vitamin E (Marin-Guzman et al., 2000).
IODINE
Iodine is required for the synthesis of thyroid hormones and the most obvious consequence of a deficiency is goiter, in which the thyroid gland enlarges as it tries to compensate for the lack of thyroid hormone production.
In the pig, reproductive failure and weak and unusually large newborn piglets are the outstanding manifestations of I deficiency. The iodine content of animal feedstuffs is extremely variable. Plants grown in regions with iodine deficiency, such as the exfoliated granite soils in the inlands of most continents, are often deficient in iodine. Cereals and oilseed meals are poor sources of I, whereas fish meal is an excellent source. On the other hand, when iodised salt (3 g KI/kg) or marine salt are used as dietary Na sources, there is no need for additional I.
CHROMIUM
Chromium has been considered by many nutritionists to be an essential nutrient for humans and animals, but clinical symptoms of deficiency have never been reported in intensive pig production. Chromium is part of the glucose tolerance factor, which enhances sensitivity of tissues to insulin and facilitates glucose uptake and utilisation by cells. There is some evidence that additional dietary Cr might be beneficial for people undergoing physical or metabolic stress, because stress and disease increase urinary excretion of Cr (NRC, 1997).
In respect to pig production, several authors have shown that organic Cr at levels of 100 to 200 ppb in the diet improves nutrient digestibility (Kornegay et al., 1997), increases muscling and decreases fatness in pigs (Boleman et al., 1995). In addition, it has been shown that organic Cr at levels of 200 ppb and beyond increase conception rate and number of pigs born alive (Lindeman et al., 2004). The NRC (1997) study found that Cr supplementation increased carcass leanness in 9 of 24 experiments and decreased carcass fat in 11 of 26 experiments. However, the beneficial effects of Cr are elusive and in consequence the NRC (1998) does not make any recommendation on dietary Cr supplementation for swine. Therefore, the decision to use supplemental Cr in pig diets must be based on confidence in the quality of the Cr product used.
COBALT
The only known function of Co is its participation in metabolism as a component of vitamin B12. Mammalian tissues lack the enzyme needed to incorporate Co to produce vitamin B12, and evidence of Co deficiency as distinct from vitamin B12 deficiency has never been described for the pig. Therefore, the inclusion of Co in the trace mineral premix is only justified when the pig has free access to faeces, since vitamin B12 can be manufactured by the hindgut microflora, as might happen in traditional open air pig production systems (free-range Iberian pigs).
Mineral contamination of the soil and the environment
The impact of intensive pig production on the environment is the major factor limiting the expansion of the industry in the EU-25 and affects the attitude of the general public towards farming. Water and soil pollution from high levels of P and other nutrients in pig manure are major issues in animal agriculture and a lack of control may adversely affect the development of the industry (Sharpley, 1999). Traditionally, the P content of the manure was viewed as a resource to supply nutrients for crop fertilisation. Increased specialisation and concentration of livestock and crop production in specific areas has led to the net export of nutrients from major crop-producing countries to regions with a high concentration of animal farming. As a result, cost of production has decreased worldwide but manure has become a contaminant rather than a fertiliser in many areas of the world.
Pig production has increased steadily in EU-15 for the last 30 years and 205.8 million pigs were slaughtered in 2004. Pig production is concentrated in specific countries which must face environmental problems related to a surplus of animal manure. In Table 12 we offered data on pig populations and concentrations in selected EU-25 countries. In Denmark, the number of slaughter pigs in 2004 was 22.9 million, which were produced in a 43,100 km2 area. Therefore, Denmark produced 531 pigs/km2 and slaughtered 4.24 pigs per each existing Dane. In the past, the figure for production of pigs in some areas of the Netherlands was over 2,600 animals/km2. It is worth noticing that the figures for pig density correspond to total area and not only agricultural area. For example, pig density in France is 48 pigs/km2 of total surface area, but is 88 pigs/km2 of agricultural area (Dourmad et al., 1999).
Table 12.Pig population in selected countries of the European Union-25 (2004). 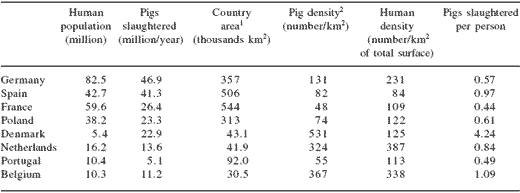
1Refers to total area of the country, not to agricultural area exclusively
2Number of pigs/km2 of total surface.
In the past, there was little pressure to decrease mineral excretion, so pig producers have typically overfed minerals to provide a safety margin. However, excess dietary mineral increases mineral burden on the environment and in consequence, the strategy is no longer desirable.
Several techniques have been proposed to reduce contamination by P and other minerals including manure management, proper litter application, moving manure from surplus to deficit areas, finding alternative uses for manure and improving conservation practices.
Probably, at least from the consumer point of view, dietary manipulation is the most cost effective tool to reduce mineral contamination (CAST, 2002; Knowlton et al., 2004). Therefore, some nutritional means of reducing mineral excretion in pig manure will be briefly described. The amount of P and other minerals excreted depends on three major factors: 1) amount consumed, 2) the efficiency of utilisation and, 3) the amount of endogenous mineral. Endogenous losses are quite constant under practical feeding conditions and therefore, to reduce excretion we need to minimise consumption and improve the efficiency of dietary mineral use. The nutritional strategies that can be used to reduce P concentration in pig manure include: 1) phase feeding, 2) formulation with digestible P values, 3) avoidance of interactions among minerals, 4) use of specific enzymes, additives, and novel seeds with low phytate content, and 5) accurate determination of ingredient composition and pig needs. CAST (2002) indicated that by implementing today's technologies and continuing research and education programs, it is reasonable to expect a 50 to 60% decrease in P excretion in swine operations in the next five years. The CAST committee also indicated that greater responses may be possible by increasing the digestibility and nutrient profile of grain resources being developed genetically. In fact, Jongbloed et al. (1997) estimated that P excretion per growing pig in the Netherlands was reduced by 50% between 1973 and 1995 because of improvements in the understanding of P availability and P requirements and the use of exogenous phytase and monocalcium phosphate. A summary of the estimated reduction of P in manure by manipulation of feeding programs and diets is shown in Table 13.
Table 13.Estimated reduction in P in pig slurry by manipulating the diet and the feeding program.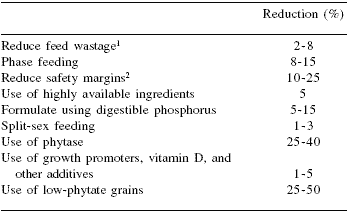
1Pelleting and feeder design
2Formulating closer to requirements and reducing variability by
quality control
PHASE FEEDING
Phosphorus requirements vary with age, sex, and physiological status of the pig. With increasing age, P needs increase but the required P content of the diet decreases because of the higher feed intake. Therefore, to minimise P losses, continuous changes in diet composition are needed. Phase feeding programs are relatively easy to implement and are one of the main tools available to reduce P excretion. In consequence, as many feeds as possible should be produced. Taking into account economics and logistics, a minimum of three feeds are recommended for pigs from 20 to 120 kg. Knowlton et al. (2004) estimated that phase feeding could reduce P excretion in growing-fattening pigs by 12.5%. If animals are grouped by gender, as well as by age, the reduction in P excretion is even greater, because gilts consume less feed and are leaner than barrows at the same body weight. Therefore, the dietary P concentration can be reduced in diets for barrows compared to gilts or entire males. However, many pig producers utilise a single feed from 20 kg to slaughter.
Two main reasons for this practice are the high percentage of farms keeping piglets from birth to slaughter (different ages in same barn) and the logistical problems of handling more than one feed on small farms.
McGlone (2000) indicated that the withdrawal of P, Ca, and trace minerals from pig diets for the last 30 days of the fattening period had no adverse effects on growth or carcass quality. Therefore, the mineral content of the finishing feed can be decreased sharply, reducing cost of production and diminishing the environmental impact of mineral excretion.
USE OF DIGESTIBLE VALUES FOR MINERAL BIOAVAILABILITY
The high mineral content of pig manure is attributable to several factors, but the main reason is the poor availability of the sources used. The majority of trace minerals ingested by domestic animals (up to 99%) are not retained and appear in faeces and urine (Nys, 2001).
On the other hand, livestock utilise P inefficiently, excreting 60 to 80% of that consumed. Therefore, the majority of the minerals from the feed remain on the farm, rather than being exported in meat. To reduce the trace mineral and P excretion of pigs by half is technically a simple task.
The concept of availability has been well accepted in the area of animal nutrition. Nutrients in feedstuffs are incompletely digested and metabolised by the organism and therefore, total values for element concentrations are of relatively little interest. Availability of minerals varies widely across ingredients. Subsequently, the use of available rather than total element values in feed formulation will allow a better match to pig needs and will help reduce nutrient excretion. However, the evaluation of digestibility of mineral ingredients is time consuming and requires sophisticated equipment, trained personnel, and funds. Thus, the available data, especially for ingredients of local origin and for pigs at the end of the fattening period, is scarce (Dourmad et al., 1999).
The use of digestible values is particularly important for P because its economic and environmental impact is highest among minerals. Improving the utilisation of supplemental mineral phosphates is an area of concern because under practical conditions close to half of the P in the excreta is derived from undigested mineral phosphates. The total excretion of P can be reduced by using more digestible sources (monocalcium phosphate vs dicalcium phosphate vs defluorinated rock phosphates vs plant phytates), avoiding interactions with other nutrients, mainly Ca, and incorporating adequate enzymes, mainly phytases, into the feed.
For determining the availability of P for pigs, different systems are used. The two main approaches are the slope ratio assay (relative values with respect to a standard source; used in the USA) and the digestibility assay in which values are obtained using in vivo studies conducted at low levels of P intake (absolute values; used in many European countries). The European approach is probably a preferred method for determination of the true biological value of P from ingredients and provides a better ranking of commercially available phosphates (Eeckhout et al., 1995). The majority of the studies concerning P utilisation are related to phytate-bound P, but greater emphasis should be directed towards the utilisation of dietary inorganic P. Phosphates produced by reacting phosphoric acid with limestone (monodicalcium phosphates) and phosphates produced by reacting rock phosphate with phosphoric acid and sodium carbonate (deflourinated phosphates) may have different availabilities for monogastric animals, however more work is needed in this area (Waldroup, 1999). With few exceptions, mono- and mono-dicalcium phosphates have the highest biological value, with dicalcium phosphates about 5% and defluorinated phosphates about 10% less in comparison (Tables 1 and 2). However, the methodology of ranking phosphate sources based on digestibility is not standardised throughout Europe and it is difficult to apply under practical conditions.
INTERACTIONS AMONG MINERALS
Elements with similar physical and chemical properties act antagonistically in biological systems. Mineral-tomineral interactions can occur anywhere in the food chain, but most of the important interactions take place in the gastrointestinal tract. Organic minerals can replace inorganic sources at a lower level and reduce mineral interactions (Miles and Henry, 1999). The mutual antagonism between Zn and Cu has been regarded as a prime example of competitive biological interactions between metals with similar chemical and physical properties. High Zn supply inhibits intestinal absorption, hepatic accumulation, and placental transfer of Cu, and also induces clinical signs of Cu deficiency (Fairweather- Tait, 1995). In animal production, Zn supplements might help alleviate toxicity in sheep fed high levels of Cu, but care should be taken in the use of excess Zn in monogastrics when Cu status is suboptimal. In fact, the use of pharmacological levels of Zn in weanling diets may jeopardise the Cu and the Fe status of the piglet.
Augspurger et al. (2004) pointed out the deleterious effects of Zn added at pharmacological levels on the efficacy of microbial phytase in improving P availability in weanling pigs.
The antagonism between Ca and P is probably the most important interaction between minerals from an economic point of view; with the excess of one reducing the absorption of the other. Because P supplementation is more expensive than Ca supplementation, excess Ca is of more practical concern. Typically, as the level of dietary Ca increases, the absorption of P decreases. In addition, excess Ca reduces phytase activity, bone strength, and performance of pigs, particularly in Pdeficient diets (Eeckhout et al., 1995). Therefore, it is convenient to formulate pig diets with a low Ca level.
USE OF ENZYMES, OTHER ADDITIVES, AND NOVEL INGREDIENTS
The use of enzymes in pig feeds has increased steadily over the last 15 years. The economics of enzyme use are beyond question with regard to phytase activity (Cromwell et al., 1993). Two thirds of total P in most feed ingredients of plant origin is present as phytic acid P, which is essentially indigestible for monogastrics.
However, in the presence of the phytase enzyme, phytic acid can be hydrolysed and then absorbed. The addition of phytase to pig diets not only renders phytate P available for absorption but also enhances the digestibility of Ca, Zn, and Mg. The prospect of using microbial phytase is very promising, and phytase is now routinely added to pig diets. In practice it is recommended to use 500 units of phytase/kg of pig diet to replace 1 g/kg of the inorganic P in the diet (Paditz et al., 2004). Many integrators utilising phytase have introduced the matrix of phytase as a new ingredient or simply have reduced available P (by approximately 0.9 g/kg) or digestible P (by approximately 0.7 g/kg) in relation to levels previously used.
Vitamin D3 metabolites and organic acids seem to increase the efficiency of use of P in poultry and also in pigs. Vitamin D3 is very much involved in Ca and P absorption and metabolism. Vitamin D status is more important in diets that are deficient in these two minerals or in diets in which the Ca:P ratio is too wide. On the other hand, it has been shown that dietary acidification may stimulate the digestion of minerals (Ravindran and Kornegay, 1993). A large portion of the digesta leaves the pig stomach shortly after feeding, and has a pH that is too high for optimal microbial phytase action. Feed acidification may reduce the rate of gastric emptying and lower pH, thus increasing mineral solubility, prolonging phytase activity in the gastrointestinal tract, and improving P, Mg, and Ca absorption (Kemme et al., 1999). Thus, the combination of phytase and organic acid addition may be an effective approach to enhancing mineral digestibility in pigs.
The development of grain and seed cultivars (corn, wheat, and soybean meal, primarily) with low phytate P content has provided a new tool for decreasing P in poultry manure. Ertl et al. (1998) indicated that phytate P in 'low phytate' corn was 51% more available than P in regular corn. However, many of these novel ingredients are obtained through genetic engineering techniques, which might reduce the interest in their utilisation under practical conditions.
Phosphorus contamination of soils and waterways is a major problem in pig production, but contamination by Na, Cu, and Zn is increasingly noted. Pig slurry has more than 90% moisture. Fattening pigs and sows provided with unrestricted water supplies show considerable individual variation in daily water consumption. For example, an allowance of between 15 to 20 L/day is recommended by the NRC (1998) for sows, but in practice and in summer conditions, consumption nearly doubles. One possible way to reduce the amount of slurry produced is to limit water consumption and thus, urine production. In this respect, dietary salt and K content must be controlled. Seynaeve et al. (1996) observed that sows fed 0.40% Na produced 11% more water in urine (13.9 vs 12.4 L/d, respectively) than sows fed diets containing 0.1% Na.
Nitrogen pollution of soils and ammonia contamination of the air is a major concern in pig production. Ammonia is a conversion product of urea in the urine catalysed by the enzyme urease, which is present in pig faeces. Its emission into the air from pig houses is a major problem in intensive pig production and is governed by three major factors: ammonia concentration, pH, and temperature. Slurry pH strongly affects ammonia emission. Canh et al. (1998) have found that the use of CaSO4, or CaCl2 instead of CaCO3 as a source of Ca in pig diets is an effective means of reducing ammonia emissions. Also, these authors have found that altering the dEB (Na + K - Cl) from 320 to 100 meq/kg dry matter reduces pH of urine and slurry, therefore lowering ammonia emissions.
Zinc and Cu are the two trace minerals of major concern with regard to environmental pollution. An excess of Zn is toxic to plants, and when soil Zn concentration is over 200-300 ppm, activity of the soil microflora is reduced. The slurry produced by growing/ finishing pigs fed diets containing 100 to 250 ppm Zn contains between 850 and 1,300 mg Zn/kg DM (Revy et al., 2003). Paboeuf et al. (2001) found that a reduction of diet Zn content from 150 ppm to 90 ppm reduced the content of Zn in faeces by 40%. In addition, Revy et al. (2003) indicated that a reduction in the Zn content of piglet diets from 3,000 to 150 ppm, and of fattener diets from 100 to 60 ppm will reduce the concentration of Zn in the slurry from 1,860 to 450 mg/kg DM (Table 14).
The application of manure collected from pigs fed diets containing pharmacological levels of Cu and Zn has serious environmental concerns; an excess of Cu causes undesirable effects on plant growth and will impair the activity of lagoon bacteria responsible for waste degradation. A study by Jondreville et al. (2002) demonstrated that a reduction of the Cu content from 175 ppm to 6 ppm in piglet feeds, and from 100 ppm to 4 ppm in fattener feeds will reduce the Cu content in the slurry from 911 to 31 mg/kg DM (Table 15). In addition, it has been estimated that pigs fed diets containing 250 ppm Cu for 100 to 150 days will increase Cu in liver tissue to levels of up to 400 to 500 ppm (Jondreville et al., 2002). Therefore, high levels of Cu in the diet may result in levels of Cu in pig meat and organs above that desirable for human foods.
These studies clearly show the need to reduce the trace mineral content of current diets in order to decrease environmental contamination. The trend towards reducing the mineral content of pig diets is expected to continue in the EU-25 (DOCE, 2003), a decision that might favour the use of phytases and more available sources of trace minerals such as certain sources of organic minerals (Miles and Henry, 1999).
Table 14. Estimation of soil contamination by zinc from pig slurry.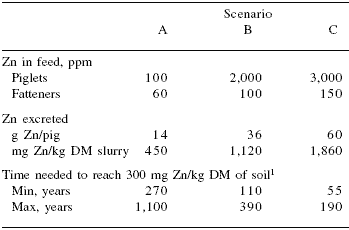
Adapted from Revy et al., 2003
1According to slurry processing practices
Table 15.Estimation of soil contamination by copper from pig slurry. 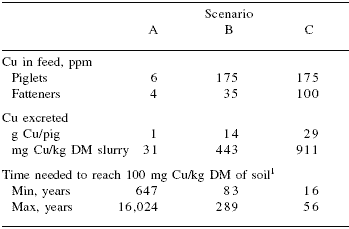
Adapted from Jondreville et al., 2002
1According to slurry processing practices
Conclusions
There is renewed interest in re-evaluating the mineral requirements of pigs and the bioavailability of commercial organic and inorganic mineral sources to better meet animal needs and to reduce whenever possible the levels of use. A reduction of dietary mineral content, especially of P, fulfils three main objectives: 1) reduces the cost of the feed, 2) avoids interactions with other minerals, and 3) decreases environmental contamination.
Dietary manipulation, although expensive, is the best alternative for reducing mineral contamination of the environment. Minimising feed wastage, phase feeding, developing feeding programs that are specific for sex and strain of the animal, and use of phytases are good strategies. In order to implement these strategies a better understanding of pig requirements according to physiological stage, and a more precise knowledge of the composition and biological value of the ingredients and mineral sources used in feeds is required.
References
Adeola, O., B.V. Lawrence, A.L. Sutton and T.R. Cline. 1995. Phytase-induced changes in mineral utilization in zinc-supplemented diets for pigs. J. Anim. Sci. 73:3384-3391.
Apple, J.K., W.J. Roberts, C.V. Maxwell, C.B. Boger, T.M. Fakler, K.G. Friesen and Z.B. Johnson. 2004. Effect of supplemental manganese on performance and carcass characteristics of growing-finishing pigs. J. Anim. Sci. 82:3267-3276.
ARC. 1981. The Nutrient Requirements of Pigs. Agricultural Research Council. CAB, Slough, UK.
Ashida, K.Y., A. Tamura, T. Matsui, H. Yano and T. Nakajima. 1999. Effect of dietary microbial phytase on zinc bioavailability in growing pigs. J. Anim. Sci. 70:306-311.
Augspurger, N.R., J.D. Spencer, D.M. Webel and D.H. Baker. 2004. Pharmacological zinc levels reduce the phosphorus-releasing efficacy of phytase in young pigs and chickens. J. Anim. Sci. 82:1732-1739.
Barber, R.S., R. Braude and K.E. Mitchell. 1955. Antibiotic and copper supplements for fattening pigs. Brit. J. Nutr. 9:378-381.
Boleman, S.L., S.J. Boleman, T. D. Bidner, L.L. Southern, T.L. Ward, J.E. Pontif and M.M. Pike. 1995. Effect of chromium picolinate on growth, body composition and tissue accretion in pigs. J. Anim. Sci. 73:2033-2042.
Braude, R. 1980. Twenty five years of widespread use of copper as an additive to diets of growing pigs. In: Copper in Animal Wastes and Sewage Sludge (P. L'Hermite and J. Dehandtschutter, eds). Proc. EEC Workshop, INRA Publisher. Bordeaux, France. pp 3-15.
BSAS. 2003. Nutrient Requirement Standards for Pigs. British Society of Animal Science. Penicuik, UK.
Canh, T.T., A.J.A. Aarnink, Z. Mroz, A.W. Jongbloed, J.W. Schrama and M.W.A. Verstegen. 1998. Influence of electrolyte balance and acidifying calcium salts in the diet of growing-finishing pigs on urinary pH, slurry pH and ammonia volatilisation from slurry. Liv. Prod. Sci. 56:1-13.
CAST. 2002. Animal diet modification to decrease the potential for nitrogen and phosphorous pollution. Issue paper Number 21. Council for Agricultural Science and Technology, Ames, Iowa.
Cromwell, G.L., D.L. Hall, G.E. Combs, O.M. Hale, D.L. Hundlin, J.P. Hitchcokk, D.A. Knabe, E.T. Kornegay, M.D. Lindemann, C.V. Maxwell and T.J. Prince. 1989. Effects of dietary salt level during gestation and lactation on reproductive performance of sows: a cooperative study. J. Anim. Sci. 67:374- 385.
Cromwell, G.L., T.S. Stahly, R.D. Coffey, H.J. Monegue and J.H. Randolph. 1993. Efficacy of phytase in improving the bioavailability of phosphorous in soybean meal and corn soybean meal diets for pigs. J. Anim. Sci. 71:1831-1840.
DeRouchey, J.M., J.D. Hancock, R.H. Hines, K.R. Cummings, D.J. Lee, C.A. Maloney, D.W. Dean, J.S. Park and H. Cao. 2003. Effects of dietary electrolyte balance on the chemistry of bood and urine in lactating sows and sow litter performance. J. Anim. Sci. 81:3067-3074.
Dersjant-Li, Y., M.W.A. Verstegen, A. Jansman, H. Schulze, J.W. Schrama and J.A. Verreth. 2002. Changes in oxygen content and acid-base balance in arterial and portal blood in response to the dietary electrolyte balance in pigs during a 9 hour period after a meal. J. Anim. Sci. 80:1233-1239.
DOCE. 2003. Reglamento (CE) Nº 1334/2003 de la comisión por la que se modifican las condiciones para la autorización de una serie de aditivos en la alimentación animal pertenecientes al grupo de los oligoelementos. B.O.E., Madrid.
DOCE. 2004. Reglamento 2004/C 50/01 de la comisión. Listado de los aditivos autorizados en los piensos. Publicada conforme a lo dispuesto en la letra b) del artículo 9 unvicies de la Directiva 70/524/CEE del Consejo sobre aditivos en la alimentación animal. B.O.E., Madrid.
Dourmad, J.Y., N. Guingand, P. Latimier and B. Séve. 1999. Nitrogen and phosphorus consumption, utilization and losses in pig production. France. Liv. Prod. Sci. 58:199-211.
D'Souza, D.N., R.D. Warner, B.J. Leury and F.R. Dunshea. 1998. The effect of dietary magnesium aspartate supplementation on pork quality. J. Anim. Sci. 76:104-109.
D'Souza, D.N., R.D. Warner, F.R. Dunshea and B.J. Leury. 1999. Comparison of different dietary magnesium supplements on pork quality. Meat Sci. 51:221-225.
Eeckhout, W., M. de Paepe, N. Warnants and H. Bekaert. 1995. An estimation of the minimal P requirements for growing-finishing pigs, as influenced by the Ca level of the diet. Anim. Feed Sci. Technol. 52:29-40.
Ertl, D.S., K.A. Young and V. Raboy. 1998. Plant genetic approaches to phosphorus management in agricultural production. J. Environ. Quality 27:299- 304.
Fairweather-Tait, S.J. 1995. Fe-Zn and Ca-Fe interactions in relation to Zn and Fe absorption. Proc. Nutr. Soc. 54:465-473.
FEDNA, 2003. Tablas FEDNA de composición y valor nutritivo de alimentos para la fabricación de piensos compuestos (2nd ed) (C. De Blas, G.G. Mateos and P.G. Rebollar, eds). Fundación Española para el Desarrollo de la Nutrición Animal, Madrid, Spain.
Ferket, P.R., E. Van Heugten, T.A.T.G. van Kempen and R. Angel. 2002. Nutritional strategies to reduce environmental emissions from nonruminants. J. Anim. Sci. 80(Suppl. 2):E168-182.
Giesemann, M.A., A.J. Lewis, P.S. Miller and M.P. Akhter. 1998. Effects of the reproductive cycle and age on calcium and phosphorus metabolism and bone integrity of sows. J. Anim. Sci. 76:796-807.
Hahn, J.D. and D.H. Baker. 1993. Growth and plasma zinc responses of young pigs fed pharmacological levels of zinc. J. Anim. Sci. 71:3020-3024.
Hamilton, D.N., M. Ellis, M.D. Hemann, F.K. McKeith, K.D. Miller and K.W. Purser. 2002. The impact of longissimus glycolytic potential and short-term feeding of magnesium sulfate heptahydrate prior to slaughter on carcass characteristics and pork quality. J. Anim. Sci. 80:1586-1592.
Hamilton, D.N., M. Ellis, F.K. McKeith and J.M. Eggert. 2003. Effect of level, source and time of feeding prior to slaughter of supplementary dietary magnesium on pork quality. Meat Sci. 65:853-857.
Haydon, K.D. and J.W. West. 1990. Effect of dietary electrolyte balance on nutrient digestibility determined at the end of the small intestine and over the total digestive tract in growing pigs. J. Anim. Sci. 68:3687- 3693.
Hill, M.G., D.C. Mahan, S.D. Carter, G.L. Cromwell, R.C. Ewan, R.L. Harrold, A.J. Lewis, P.S. Miller, G.C. Shurson and T.L. Veum. 2001. Effect of pharmacological concentrations of zinc oxide with or without the inclusion of an antibacterial agent on nursery pig performance. J. Anim. Sci. 79:934-941.
Hill, M.G., G.L. Cromwell, T.D. Crenshaw, C.R. Dove, R.C. Ewan, D.A. Knabe, A.J. Lewis, G.W. Libal, D.C. Mahan, G.C. Shurson, G.C. Shurson, L.L. Southern and T.L. Veum. 2000. Growth promotion effects and plasma changes from feeding high dietary concentrations of zinc and copper to weanling pigs (regional study). J. Anim. Sci. 78:1010-1016.
Hypor. 2000. Requerimientos nutricionales para cerdos. Euribrid España, S.A. La Coruña, Spain. Ib Hagsten, T.R. Cline, T.W. Perry and M.P. Plumlee. 1976. Salt supplementation of corn-soy diets for swine. J. Anim. Sci. 42:12-15.
INRA-AFZ. 2002. Tables de composition et de valeur nutritive des matières premières destinèes aux animaux d'élevage: Porcs, volailles, bovins, ovins, caprins, lapins, chevaux and poissons (D. Sauvant, J.M. Perez and G. Tran, eds). INRA, Paris, France.
Iowa State University. 1996. Life cycle swine nutrition. PM-489I. Iowa State University Extension Service. Ames, IA, USA.
Jondreville, C., P.S. Revy, A. Jaffrezic and J.Y. Dourmad. 2002. Le cuivre dans l'alimentation du porc: oligoélément essential, facteur de croissance et risqué potential pour l'homme et l'environnement. INRA Prod. Anim. 15:247-265.
Jongbloed, A.W., N.P. Lenis and Z. Mroz. 1997. Impact of nutrition on reduction of environmental pollution by pigs: An overview of recent research. Vet. Q. 19:130-134.
Kansas State University. 1997. Starter pig recommendations. 1997. MF 2300. Agricultural Experiment Station and Cooperative Extension Service. Kansas State University, Manhatan, KA, USA.
Kansas State University. 1997a. Breeding herd recommendations for swine. 1997. MF 2302. Agricultural Experiment Station and Cooperative Extension Service. Kansas State University, Manhatan, KA, USA.
Kansas State University. 1997b. Growing-finishing pig recommendations. 1997. MF 2301. Agricultural Experiment Station and Cooperative Extension Service. Kansas State University, Manhatan, KA, USA.
Kats, L.J., J.L. Nelssen, R.D. Goodband., M.D. Tokach, K.G. Friesen, K.Q. Owen and B.T. Richert. 1994. Effect of manganese level and source on growth performance and carcass characteristics of finishing pigs. J. Anim. Sci. 72(Suppl. 1):217(Abst.).
Kemme, P.A., A.W. Jongbloed, Z. Mroz, J. Kogut and A.C. Beynen. 1999. Digestibility of nutrients in growing-finishing pigs is affected by Aspergillus niger phytase, phytate and lactic acid levels. 2. Apparent total tract digestibility of phosphorus, calcium and magnesium and ileal degradation of phytic acid. Liv. Prod. Sci. 58:119-127.
Kim, Y.Y. and D.C. Mahan. 2001a. Prolonged feeding of high dietary levels of organic and inorganic selenium to gilts from 25 kg body weight through one parity. J. Anim. Sci. 79:956-966.
Kim, Y.Y. and D.C. Mahan. 2001b. Comparative effects of dietary levels of organic and inorganic selenium on selenium toxicity of growing-finishing pigs. J. Anim. Sci. 79:942-948.
Knight, C.D., K.C. Klasing and D.M. Forsyth. 1983. E. coli growth in serum of iron dextran-supplemented pigs. J. Anim. Sci. 57:387-395.
Knowlton, K.F., J.S. Radcliffe, C.L. Novak and D.A. Emmerson. 2004. Animal management to reduce phosphorus losses to the environment. J. Anim. Sci. 82(Suppl. 1):E173-E195.
Kornegay, E.T., H.R. Thomas and T.N. Meacham. 1973. Evaluation of dietary calcium and phosphorus for reproducing sows housed in total confinement on concrete or in dirt lots. J. Anim. Sci. 37:493-500.
Kornegay, E.T., B.G. Diggs, O.M. Hale, D.L. Handlin, J.P. Hitchcook and R.A. Barczewski. 1985. Reproductive performance of sows fed elevated calcium and phosphorus levels during growth and development. J. Anim. Sci. 61:1460-1466.
Kornegay, E.T., Z. Wang, C.M. Wood and M.D. Lindemann. 1997. Supplemental chromium picolinate influences nitrogen balance, dry matter digestibility and carcass traits in growing-finishing pigs. J. Anim. Sci. 75:1319-1323.
Kuhn, G., A. Nowak, E. Otto, V. Albrecht, B. Gassmann, E. Sandner, H. Przybilski and L. Zahn. 1981. Untersuchngen zur Beeiflussung der Fleischbeschaffenheit mit Hilfe spezieller Behandlungsverfahren beim Schwein. 1. Mitteillung: Einfluss von Stress und praventiver Magnesium zufutterung auf ausgewahlte Parameter des Schlachtkorperwertes und des Blutserums. Archives für Tierzuchtzg Berlin 24:217-225.
Lahucky, R., U. Küchenmeister, I. Bahelka, D. Vasicek, T. Liptaf and K. Ender. 2004. The effect of dietary magnesium oxide supplementation on postmortem 31P NMR spectroscopy parameters, rate of Ca2+ uptake and ATPase activity of M. longissimus dorsi and meat quality of heterozygous and normal or malignant hyperthermia pigs. Meat Sci. 67:365-370.
Liebholz, J.M., V.C. Speer and V.W. Hays. 1962. Effect of dietary manganese on baby pig performance and tissue manganese levels. J. Anim. Sci. 21:772-776.
Lindemann, M.D., S.D. Carter. L.I Chiba, C.R. Dove, F.M. LeMieux and L.L. Southern. 2004. A regional evaluation of chromium tripicolinate supplementation of diets fed to reproducing sows. J. Anim. Sci. 82:2972-2977.
Mahan, D. 1982. Dietary calcium and phosphorus levels for weanling swine. J. Anim. Sci. 54:559-564.
Mahan, D.C., T.D. Wiseman, E. Weaver and L. Russell. 1999. Effect of supplemental sodium chloride and hydrochloric acid added to initial starter diets containing spray-dried blood plasma and lactose on resulting performance and nitrogen digestibility of 3-week-old weaned pigs. J. Anim. Sci. 77:3016-3021.
Mahan, D. 2004. The role of selenium and Sel-Plex® in sow reproduction. In: Nutritional Biotechnology in the Feed and Food Industries, Proceedings of Alltech 20th Annual Symposium (T.P. Lyons and K.A. Jacques, eds). Nottingham University Press, UK, pp. 131-139.
Marin-Guzman J., D.C. Mahan and J.L. Pate. 2000. Effects of dietary selenium and vitamin E on spermatogenic development in boars. J. Anim. Sci. 78:1537-1543.
Mateos, G.G., D. García and E. Jiménez. 2004. Microminerales en alimentación de monogástricos. Aspectos técnicos y consideraciones legales. Fedna 20:275-323.
Mateos, G.G., R. Lázaro, J.R. Astillero and M. Pérez. 2005. Trace minerals: what text books don't tell you. In: Re-Defining Mineral Nutrition (J.A. Taylor- Pickard and L.A. Tucker, eds). Nottingham University Press. UK, pp 21-62.
Mavromichalis, I., J.D. Hancock, I.H. Kim, B.W. Senne, D.H. Kropf, G.A. Kennedy, R.H. Hines and K.C. Behnke. 1999. Effects of omitting vitamin and trace mineral premixes and(or) reducing inorganic phosphorous additions on growth performance, carcass characteristics and muscle quality in finishing pigs. J. Anim. Sci. 77:2700-2708.
McGlone, J.J. 2000. Deletion of supplemental minerals and vitamins during the late finishing period does not affect pig weight gain and feed intake. J. Anim. Sci. 78:2797-2800.
Miles, R.D. and P.R. Henry. 1999. Relative trace mineral bioavailability. Proc. California Anim. Nutr. Conf. Fresno, CA, USA, pp. 1-24.
Nebraska (University of) and South Dakota State University. 2000. Swine Nutrition Guide EC 95-273- C. Nebraska Cooperative Extension Service. Lincoln, NE, USA.
Nimmo, R.D., E.R. Peo, B.D. Moser and A.J. Lewis. 1981. Effect of level of dietary calcium-phosphorus during growth and gestation on performance, blood and bone parameters of swine. J. Anim. Sci. 52:1330- 1342.
NRC. 1988. Nutrient Requirements of Swine. 9th Rev., National Academy Science, Washington DC, USA.
NRC. 1997. The Role of Chromium in Animal Nutrition. National Academy Science, Washington DC, USA.
NRC. 1998. Nutrient Requirements of Swine. 10th Rev., National Academy Science, Washington DC, USA.
Nys. Y. 2001. Oligo-éléments, croissance et santé du poulet de chair. INRA Prod. Anim. 14:171-180.
Osborne, J.C. and J.W. Davis. 1968. Increased susceptibility to bacterial endotoxin of pigs with irondeficiency anaemia. J. Amer. Vet. Medical Soc. Assoc. 152:1630-1632.
Paboeuf, F., C. Calvar, B. Landrain and H. Roy. 2001. Impact de la reduction des niveaux alimentaires en matières azotée totale, en phosphore, en cuivre et en zinc sur les performances et les rejets des porcs charcutiers. J. Rech. Porcine en France. 33:49-56.
Paditz, K., H. Kluth and M. Rodehutscord. 2004. Relationship between graded doses of three microbial phytases and digestible phosphorus in pigs. J. Anim. Sci. 78:429-438.
Patience, J.F. and R.K. Chaplin. 1997. The relationship among dietary undetermined anion, acid-base balance and nutrient metabolism in swine. J. Anim. Sci. 75:2445-2452.
Patience, J.F., R.E. Austic and R.D. Boyd. 1987. Effect of dietary electrolyte balance on growth and acidbase status in swine. J. Anim. Sci. 64:457-466.
PIC. 2002. Recomendaciones nutricionales para porcino. Boletín Técnico Nº 24. Barcelona, Spain. Ravindran, V. and E.T. Kornegay. 1993. Acidification of weaner pig diets: A review. J. Sci. Food Agric. 62:313-322.
Rayman, M.P. 2002. The argument for increasing selenium intake. Proc. Nutr. Soc. 61:203-215.
Revy, P.S., C. Jondreville, J.Y. Dourmad and Y. Nys. 2003. Le zinc dans l'alimentation du porc: oligoélément essential et risque potential pour l'environnement. INRA Prod. Anim. 16:3-18.
Schell, T.C. and E.T. Kornegay. 1996. Zinc concentration in tissues and performance of weanling pigs fed pharmacological levels of zinc from ZnO, Zn-Methionine, Zn-Lysine, or ZnSO4. J. Anim. Sci. 74:1584-1593.
Shannon, M.C. 2005. Piglet diets: can we manage without zinc oxide and copper sulphate?. In Trace minerals: what text books don't tell you. In: Re- Defining Mineral Nutrition (J.A. Taylor-Pickard and L.A. Tucker, eds). Nottingham University Press, UK, pp. 75-87.
Sharpley, A. 1999. Agricultural phosphorus, water quality and poultry production: are they compatible? Poult. Sci. 78:660-673.
Seynaeve, M., R. de Wilde, G. Janssens and B. De Smet. 1996. The influence of dietary salt level on water consumption, farrowing and reproductive performance of lactating sows. J. Anim. Sci. 74:1047- 1055.
Surai, P.F. 2003. Selenium - Vitamin E interactions: does 1 + 1 equal more than 2? In: Nutritional Biotechnology in the Feed and Food Industries, Proceeding of Alltech's 19th Annual Symposium (T.P. Lyons and K.A. Jacques, eds). Nottingham University Press, UK, pp. 51-58.
Underwood, E.J. and N.F. Suttle. 1999. The Mineral Nutrition of Livestock. Cabi Publishing (3rd ed). Wallingford, UK. Waldroup, P.W. 1999. Nutritional approaches to reducing phosphorus excretion by poultry. Poult. Sci. 78:683-691.
Yen, J.T., G. Pond and R.L. Prior. 1981. Calcium chloride as a regulator of feed intake and weight gain in pigs. J. Anim. Sci. 52:778-782.
Authors: G.G. MATEOS, R. LÁZARO, D.G. VALENCIA and B. VICENTE
Departamento de Producción Animal, Universidad Politécnica de Madrid, Madrid, Spain





.jpg&w=3840&q=75)