Introduction
The use of high quality boar semen is critical to obtaining desired herd fertility goals. When using artificial insemination (AI) in a breeding program, the semen supplier becomes an external input which can effectively drive a customer’s herd reproductive performance. Depending upon circumstance, this input can either aid in or interfere with achieving herd production goals. Abrupt increases in regular return rates leading to repeat services may serve as early indicators of semen quality issues. End-point data suggestive of semen quality problems can include decreases in farrowing rates and average total born per litter, and/or increases in litter scatter. Expectations of fluctuations in this end-point data can be quite variable, driven not just in the etiology of the semen quality issue, but also by herd management factors which may exacerbate the problem. When reviewing the literature, earlier studies (1) have reported mean reductions of 17% and 1.2 piglets born alive due to poor AI semen quality on farms (N=37) experiencing reproductive problems. A more recent retrospective assessment of poor reproductive performance in 600 herds (250-5,000 sows) that use AI identified semen quality issues as the cause of decreased reproductive performance in 120 (33.3%) of the herds (2).
When assessing the impact of a boar stud’s contributions to herd fecundity, a process control strategy is employed to identify and eliminate special causes of variation. The diagnostician should obtain both pertinent pre-use data on the extended semen product originating from the stud, along with the post-use fertility data from the sow farm(s). Temporal overlayment of these data often aids in narrowing down the time frame of a more detailed diagnostic focus. Assurances to the accuracy and completeness of information from the stud and sow farm(s) are critical to diagnostic success. This report highlights some of the more recent issues which have been identified as leading to disturbances to semen quality and subsequent herd fertility through recent field investigations.
Initial Ejaculate Quality
All progressive boar studs today employ some type of assessment of a freshly collected ejaculate which is intended for distribution and use in an AI program. The most common ejaculate parameters assessed at a stud include ejaculate volume, color, odor, sperm motility, sperm morphology, and sperm concentration. Ejaculate color/odor, sperm motility and sperm morphology are qualitative assessments, whereas ejaculate volume and sperm concentration data are quantified in order to determine eligible dose numbers from the ejaculate. Minimum requirements of fresh boar semen for use in AI programs have been established (3,4). Color and odor are very quick and simple procedures which are used to identify the presence of certain spermicidal contaminants (i.e., urine, blood, diverticulum fluid). The remaining qualitative parameters of sperm motility and sperm morphology are typically assessed to verify that ejaculates meet or exceed a desired threshold level prior to inclusion in a commercial AI dose. Current recommendations suggest at least ≥80% progressively motile sperm in conjunction with having ≥75% morphologically normal sperm cells (3,4), and are typically defined in a contract between the sow farm and stud. Any investigation should confirm that threshold quality parameters are being met, along with affirming that the stud uniformly applies consistent, objective processes using validated techniques.
Table 1. General stud hygiene and sanitation recommendations (9,10).
Personnel
1. Application of good hand hygiene, including appropriate washing and use of protective gloves, should be practiced throughout all areas of the stud
2. Personnel should avoid contact of bare hands with materials which can later come into contact with the semen or extender
3. Personnel with upper respiratory infections should be cognizant of and avoid contamination of materials, semen or extender through aerosolization during sneezing or coughing
4. Caps/hair nets can be of value if worn by personnel performing the semen collection process and by laboratory personnel as an aid in minimizing hair and dander as a contamination source
5. Clean protective garments and shoes ⁄ boots, provided on site by the stud, should be available for use by all stud personnel
Animal housing ⁄ handling
1. Animal housing should regularly be sanitized, including removal of organic material and application of a broadspectrum disinfectant
2. Trimming of hair surrounding the preputial orifice should occur on an as needed basis to eliminate the accumulation of organic matter at this site and its inadvertent introduction into the ejaculate during semen collection
3. The ventral abdomen should be clean and dry prior to commencing with semen collection
4. Cleaning of the preputial opening and surrounding area with a single-use disposable wipe should be considered if the area is wet and ⁄ or has organic material present
5. Preputial fluids can contain high numbers of bacteria, therefore, these fluids should be evacuated immediately prior to the semen collection process
6. When collecting semen, the collector should position the penis in such a way as to minimize gravitational contamination of the semen collection vessel with preputial fluids
7. If performing gloved hand semen collection, divert the pre-sperm fraction from the semen collection vessel to reduce ejaculate bacterial load
8. The semen collection area and any collection equipment should be thoroughly cleaned and disinfected at the end of each collection day, with adequate time to dry.
Laboratory
1. Encourage single-use disposable products when economically feasible to minimize cross-contamination
2. When using reusable laboratory materials (i.e. glassware, plasticware, plastic tubing, containers, etc.) which cannot be heat ⁄ gas sterilized or boiled, clean products using a laboratory-grade detergent (residue-free) with water, followed by a distilled water rinse, and lastly through a 70% alcohol (non-denatured) rinse. Allow sufficient time and proper ventilation for complete evaporation of residual alcohol. Rinse reusable with semen extender prior to first use
3. Laboratory purified water should be checked on a minimum monthly basis if in-house or by lot if outsourced. Any bacterial growth should be considered significant and appropriate action taken to identify and eliminate the contaminant source
4. Disinfect countertops and contaminated lab equipment at end of processing day with a residue-free detergent and rinse
5. Floor should be mopped at end of day with a disinfectant
6. Break down bulk products into smaller, daily use quantities immediately after opening
7. Ultraviolet lighting can be installed to aid in sanitizing reusables and laboratory surfaces; however, safety precautions should be integrated to prevent exposure to personnel
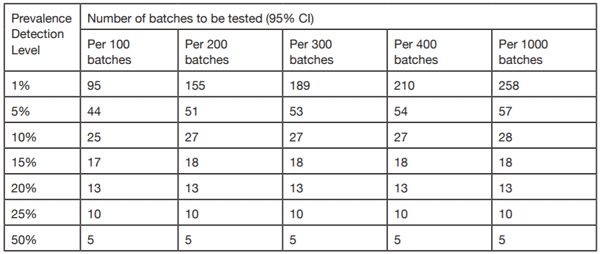
Table 2. Number of samples to be QC tested (95% CI) relative to the proportion of ejaculates/batches processed at a desired prevalence detection level (11).
Product Contaminants
Adoption of AI in the swine industry has been broad, with this reproductive technology now having a global footprint. As a maturing market, the competition by the suppliers of materials for boar studs remains diverse and intense. Classic marketing strategies by AI materials suppliers have included being a low cost provider or purveyor of quality products. The former drives supplier revenue through sales volume, while the latter achieves revenue through the production of consistent, quality products. In an ideal situation, the optimal materials supplier to a boar stud would be one that combines both cost reduction and product quality improvement. The supplier should be able to demonstrate to the stud acceptable supply chain practices which assure not only consistency and quality of raw ingredients, but also an appropriate level of in vitro and in vivo screening processes on the final product prior to distribution.
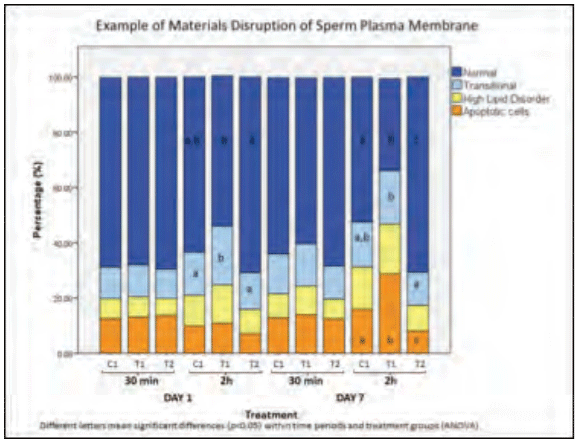
Reports of reduced litter size (total born) across multiple sow farms initiated a field investigation. Multiple (N=3) boar studs sourced the extended semen used on these farms. Sow farm reproductive records were collected and assessed, amounting to ~130,000 services. Of these services, 77% were performed using extended semen in blister bags, with the remaining services using extended semen held in tubes. An initial data review showed distinct fecundity differences based upon whether blister bags or tubes were used for breeding. Retained blister bags and tubes were tested using standard spermiogram analyses (e.g., sperm motility and morphology) over a 7-day storage period, with no differences observed between the receptacles. Subsequently, additional testing was developed in order to assess sperm cell plasma membrane fluidity and applied to samples stored in blister bags and tubes. Time dependent degradation in plasma membrane fluidity was found to occur in association with samples held in the blister bags (Figure 1), suggesting a deleterious effect of material on the sperm. Subsequent work by others (12) identified the leaching of specific compounds (i.e., BPA, BADGE, PVC, phthalates) from the multilayer blister bags which perturbed sperm cells.
Figure 1. Disruption to sperm plasma membrane fluidity associated with exposure to select plastics. Note variation in percentage of sperm exhibiting transitional fluidity between the control (C1), treatment 1 (T1) and 2 (T2) groups.
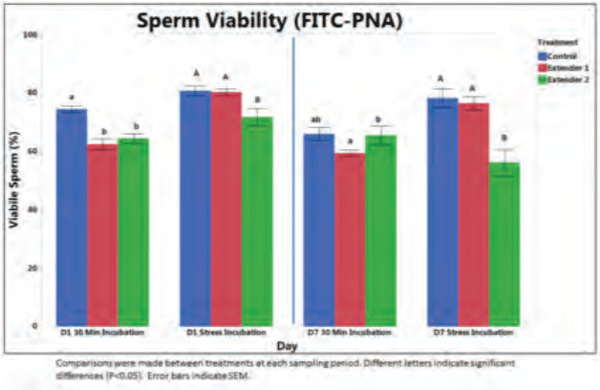
In 2015, multiple sow farms reported a reduction in litter sizes. Further investigation using data obtained from 36 breeding farms confirmed an average decrease of 0.7 less piglets/litter, starting breeding week 13. Focusing on the time period that reduced litter sizes commenced, the stud’s material supply records were assessed, identifying that new semen extender product lots were incorporated into extended semen production. Further in vitro testing of the extender was performed, along with comparison to other semen extenders. Boar semen samples held in the extender in question were found to be stress sensitive, resulting in lower numbers of acrosome intact, viable sperm (Figure 2). Changes in extender choice within the production system lead to a return to normal reproductive performance on the sow farms.
Figure 2. The effect of semen extender on percentages of acrosome-intact, viable (AIV) sperm over time. Under stress incubation, Extender 2 has less (P< 0.05) AIV sperm than either currently used extender (Control) or test extender 1.
Conclusion
Semen quality is a vital component to achieving sow herd fecundity goals. Proactively, boar studs need to have protocols in place which objectively assure that the extended semen product meets or exceeds quality parameters and consistency. Boar studs should screen AI material suppliers to assure that they abide by acceptable supply chain practices which support product safety, consistency and traceability. Not only should these practices be able to assure proper design, monitoring, and control of manufacturing processes and facilities, but they should also include an appropriate level of in vitro and in vivo testing which supports quality and consistency of the final product prior to distribution. Detailed recordkeeping of all material/supply lots used at a stud, in conjunction with timely feedback and accessibility to sow farm reproductive records, is crucial to both the stud’s quality assurance program and to delineating the contributions the stud has to a sow farm’s fecundity goals.
Presented at the 24th International Pig Veterinary Society Congress. For information on the next edition, click here.