Health Management with Reduced Use of Antibiotics in Pig Production
Published: November 29, 2013
By: James Pettigrew (University of Illinois) and Rodney B. Baker (North Carolina State University)
The world's food production system has responded magnificently to the need to dramatically increase production during the last few decades, to supply the growing human population of the world. But the challenge continues, driven by both the projected increase in population and the wonderful improvement in living standards and diet quality that will be the fortunate lot of many people in the developing world. The need to continue to increase food production with limited resources places the onus squarely on the livestock industry to increase both efficiency and production.
Protecting the health of animals in future livestock production systems will be paramount to successfully meeting the food needs of the global human populations. Disease elimination and exclusion of economically important agents have proven difficult at best, requiring adoption of a multitude of functional management opportunities. One important component of health maintenance has long been the use of antibiotics. Thus, a high level of efficiency and productivity requires healthy animals. Against this backdrop, other ethical and economic concerns also focus attention and resources on keeping animals healthy. Keeping animals healthy is a difficult challenge in any circumstances, but the difficulty is magnified by limitations on the use of such a powerful health technology as antibiotics.
The convention has been historically to consider three separate roles of antibiotics in animal production: therapeusis, prophylaxis and growth promotion. In practice, the boundary between use for prophylaxis and growth promotion has often been indistinct, so when use of antibiotics for 'growth promotion' is halted, there is often an increase in disease (McEwen et al., 2003). The reasons for reduction of antibiotic use in animal production may be strong and valid, but the implementation of such reduction has dramatic impacts on the maintenance of animal health.
We believe the appropriate reaction to such a change is to re-evaluate the entire health management system. Our focus is on the pig industry, where the health management programme, as we view it, centres on such characteristics of the production system as multi-site production, pig flow and weaning age. Other important components include biosecurity, sanitation, vaccines and dietary factors. We reject the notion that we should simply seek 'alternatives to antibiotics' as too narrow, but there is strong evidence that some feed ingredients and other characteristics of the feeding system can be important components of a health management programme.
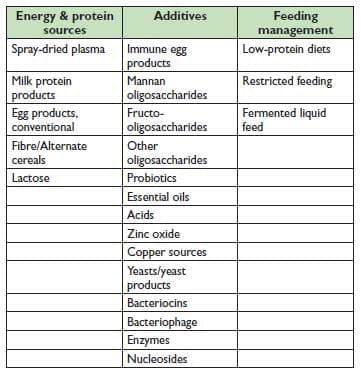
Table 27.1. An incomplete list of potential dietary technologies to improve pig health and productive performance.
This chapter will focus first on the rich supply of feedbased health technologies now available for use by the industry, and then will consider the practical and successful application of a wide range of health technologies for pigs with reduced antibiotic use.
Feed-based Health Technologies
The group of technologies considered herein is restricted to dietary ingredients that have physiological activity beyond provision of bioavailable nutrients, and to formulation practices and feeding methods that similarly alter physiological conditions. Many of them are suggested to provide benefits through impacts on microbial populations in the digestive tract and/or influence on immunity, although other modes of action also fall within the scope of this discussion. Consideration of microbial populations in the digestive tract draws attention to growth and survival of pathogens, but also includes the potential importance of commensal bacteria.
The industry now has a rich supply of potential dietary technologies available for evaluation and use (Table 27.1). A thorough discussion of all of these potential dietary tools is beyond the scope of this paper, but some of them are discussed in varying depth below.
This discussion relies somewhat on the powerful but imperfect statistical approach of meta-analysis, a combination of experiments into a single statistical analysis. Meta-analysis achieves substantial experimental power through the amalgamation of several experiments, overcoming the fact that most single experiments have too little experimental power to detect some important treatment effects. It also provides an unusually broad inference space because of the range of conditions under which the several experiments are conducted.
The results of a meta-analysis reflect the experiments included, so it is important that those experiments are representative of conducted or potential experiments. A biased sample leads to biased results. That is a special problem when dealing with the published literature because scientists or journals often choose not to publish data that fail to show statistically significant differences among experimental treatments. That regrettable choice results in a bias in the refereed literature, which results in an unavoidable bias in the results of a meta-analysis based on the scientific literature. Note that this bias affects any review of the literature, but is most obvious when the review is formalised and quantitative as in the case of a meta-analysis. It likely overestimates the benefit of dietary factors in some of the cases considered here.
Spray-dried Plasma
Two meta-analyses of the impact of spray-dried plasma on the growth performance of young pigs were reported in 2001 (Coffey and Cromwell, 2001; van Dijk et al., 2001). Each found dramatic responses to plasma, with mean increases in growth rate of 25% and 27%. Such large increases in pig growth rate are rare, and the size of the impact has driven the widespread adoption of spraydried plasma in diets for weaner pigs in spite of its relatively high cost.
We have recently reviewed data that were not included in the earlier reviews, and found a mean increase in growth rate of 23% (P<0.0001) when plasma was included in the diet, in good agreement with the earlier reviews. We believe the positive bias often found in the literature is unlikely to occur in this case, largely because most of the recent studies have not been conducted to evaluate the effect of plasma, but to evaluate other products as potential 'plasma replacements'. Thus, whether there is a response to plasma may be largely irrelevant to a decision about publication. The recent data indicate that the response to plasma does not diminish with increased weaning age, and does not appear to be strongly related to the protein source the plasma replaces.
There is now strong evidence that spray-dried plasma in the diet provides protection against enteric disease caused by Escherichia coli (e.g. Bosi et al., 2001; Owusu-Asiedu et al., 2003). This development has enormous practical importance.
We still do not understand clearly the mechanisms through which dietary spray-dried plasma improves growth performance and protects against disease. However, it is useful to remember that plasma carries many physiological signals throughout the body, so it contains functional components. The most likely mechanisms relate to increased feed intake and to protection against disease.
Protective effects of plasma may derive from its immunoglobulin content (Pierce et al., 2005), from glycoprotein glycans which may block adhesion of pathogens to intestinal binding sites (Nollet et al., 1999), or from immunomodulation. Dietary plasma appears to down-regulate the inflammatory process in healthy pigs (Touchette et al., 2002), which may contribute to increased feed intake and direction of nutrients to productive functions. However, in immune-challenged pigs, dietary plasma stimulates immune function (Touchette et al., 2002), presumably providing protection.
Immune Egg Products
Hens immunised against pig pathogens produce antibodies against those pathogens and deposit them in eggs. Those eggs or their components can then be fed to pigs to provide passive immunity to the diseases in question. Selection of the most appropriate antigen and immunisation schedule appears critical to success. In most experiments published to date, antibodies have been raised to surface antigens of E. coli, including the K88 and F18 antigens.
It is now clear that this technology can be enormously effective in reducing the percentage of pigs that die or show clinical signs (e.g. Yokoyama et al., 1997; Marquardt et al., 1999), although failures to provide benefits (e.g. Chernysheva et al., 2003) must also be acknowledged. In some cases, disease was largely controlled by spray-dried plasma and there was no further benefit from immune egg products (Owusu-Asiedu et al., 2003).
Acids
Addition of organic acids to pig diets has been suggested to improve growth performance. The perceived mechanisms include reducing the pH of the stomach contents, which in turn may improve nutrient digestion and change the microbial populations in digesta. A second mechanism is that acids in undissociated form can enter bacterial cells, where they dissociate and damage or kill the cell. Different acids may have different effects.
It is common to feed combinations of acids rather than individual ones, and sometimes the combinations include inorganic as well as the more common organic acids. Unfortunately, most of the published data address single acids.
A review of a substantial body of data (M.T. Che and J.E. Pettigrew, unpublished) shows impressive increases in growth rate of 12% (P<0.001) during the first 2 weeks after weaning, 6% (P<0.001) during the first 4 weeks, 4% (P = 0.01) during the growing phase and 3% (P = 0.02) during finishing when acids are added to the diet. The likely overestimation due to bias in the literature discussed above applies to these numbers to an unknown extent. The response to acids is remarkably robust, with no detectable influence in starting pigs of weaning age, presence of animal proteins in the diet, growth rate or acid inclusion level on the size of response. There also appear to be increases in dry matter digestibility of one percentage unit (P = 0.01) and in protein digestibility of 3 percentage units (P = 0.001).
Lactose
Lactose is an easily digested carbohydrate, but it may also be a prebiotic (a compound that serves as a preferred substrate for certain bacteria and therefore encourages their proliferation in the digestive tract). It has been suggested (Partanen et al., 2001) that dietary lactose may stimulate the growth of organisms such as Lactobacilli in the stomach, that the Lactobacilli ferment the lactose to lactic acid, and the net effect may be similar to feeding lactic acid. Data from our laboratory (Palacios et al., 2004) refute that suggestion, showing that dietary lactic acid has impacts on conditions in the digestive tract that are not mimicked by lactose.
Lactose improves the growth performance of young pigs (Tokach et al., 1989) and is widely used their diets.
Mannan Oligosaccharide
Products described as mannan oligosaccharides contain mannose, but are more complex than suggested by the term, being preparations of the outer layer of the cell wall of yeast. The mannose is key to one perceived mechanism of action of this product.
Most enteric pathogens must attach to the intestinal wall in order to proliferate and cause disease; more specifically they attach to carbohydrates as the binding sites. Several pathogens, including some E. coli, attach to mannose units on the mucosal surface. It is perceived that the yeast cell wall fragment containing a mannose unit in the lumen of the intestine may bind to the pathogens, preventing the pathogens from binding to the intestinal wall. The product must survive the digestive processes and reach the lower intestine in order to function in this manner. Recent results from our laboratory (Miguel et al., 2006) confirm that a mannan oligosaccharide product changes the microbial populations in the digestive tract of young pigs.
There is also growing evidence that mannan oligosaccharide modulates the immune system (Kim et al., 2000; Shashidhara and Devegowda, 2003).
It is clear from a meta-analysis that a mannan oligosaccharide increases growth rate of young weaned pigs by about 4% (P<0.01), with the response being larger where pigs grow more slowly (Miguel et al., 2004). This metaanalysis did not rely completely on published data, so the potential bias is reduced.
Fibre
Dietary fibre consists mainly of non-starch polysaccharides, carbohydrates that are not digested by the enzymes produced by animals. Because they escape digestion in the upper digestive tract, they are available for fermentation by the microbes that inhabit the lower gut to support their proliferation. The question of whether dietary fibre is either beneficial (Wenk, 2001) or detrimental (Hopwood et al., 2004) in disease resistance is controversial.
Practical Experiences with Antimicrobial-free (ABF) Pig Production
History and Challenges
Antibiotic-free (ABF) pork, the most extreme form of reduced antibiotic use, found its place in the US grocer's meat case during the early years of the 21 century ). Its development was driven largely by perceived marketing opportunities created by consumer interest in ABF pork which, in turn, was promoted by consumer advocacy groups.
Many consumers continue to believe in the health benefits of consuming meat products from animals raised free from antimicrobial (antibiotic) exposure, in spite of a lack of clarity in the scientific literature about the amount of antibiotic resistance in human medicine caused by use of antibiotics in animal production (Phillips et al., 2004; Lusk et al., 2006; Sørum et al., 2006; Zhang et al., 2006; Duriez and Topp, 2007; Gilchrist et al., 2007; Macovei and Zurek, 2007; McMahon et al., 2007).
It was soon discovered that production of ABF pork is more expensive than production of conventional pork2). The extra cost of ABF pigs occurs not only at the production level but also within the manufacturing, marketing and advertising efforts of those companies offering ABF products to the consumer. These added costs must be justified by higher value of the 'branded' ABF pork, but to date only a few cuts of pork meat (loins, chops, etc.) have captured the anticipated added value. The burden of the added cost must be borne by the increased value of only a small proportion of the final product. Most of the pig is never recognised as a branded, value-added product.
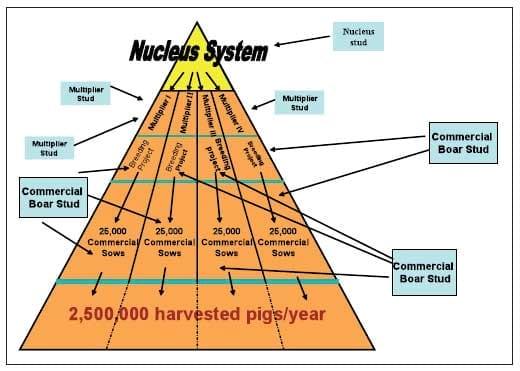
Figure 27.1. An example of a modern 'Production Pyramid' illustrating pig flow and semen inputs. Movement is always down the pyramid and never in an upwards vertical or horizontal direction. Source: Rodney B. Baker.
Pig well-being/welfare issues are likewise significant because ABF pigs typically suffer from endemic diseases at a greater intensity than conventionally reared pigs in the same production enterprise. Companies producing ABF pigs run the risk of being singled out over welfare issues by watchdog groups.
Thus far, large integrated systems have produced the vast majority of the ABF pigs produced in the United States, contrary to the expectation that ABF production would favour small producers. There are many reasons for this unintended consequence; primarily the inconsistency of supply and quality coming from the small pig farms has not met the needs of the modern food service industry.
Role of Breeding Herd Health
Producing healthy pigs is essential for competitiveness and begins with the health status of the breeding herd. Large operations typically move pigs down a production pyramid (Figure 27.1) beginning with the genetic nucleus where genetic progress is made. Pigs of this generation move to the multiplication level of production, which performs the duty of producing the replacement breeding animals (parent stock) whose offspring become the vast majority of pigs harvested for pork meat. The pyramid is similar for ABF and conventionally reared pigs. The health status of the market pigs is a result of the original health status of the nucleus level stock, as well as the deterioration of health status that occurs as pigs move down the pyramid to the different levels of production (Baker, 2006), ending at the harvest segment. This deterioration is due to lapses in knowledge or implementation of biosecurity at each step of the process, reflecting the presence of multiple weaknesses of exclusion, management and containment. All pig production systems suffer from this deterioration of health and struggle with the development and implementation of functional strategies which exclude or control pathogens. However, these strategies are especially critical when producing ABF pigs.
Production systems of all sizes struggle to maintain animal health. Small farms may claim innate biosecurity advantages but typically lack the veterinary sophistication, expertise, production infrastructure and motivation required to capture this opportunity. Small producers have also failed to meet the supply, uniformity and meat quality needs of the food service industry, a failure that to date has limited their sustainability in the market place.
Role of Growing Pig Health
Most of the significant added costs of ABF production arise from 1) interventions to maintain pig health, and 2) the poor health resulting from the inadequacy of those interventions. Although there are many useful tools for maintaining health, usage of additional immune stimulation products in the growing pig is common. Most efforts are directed at controlling the effects of population endemic bacterial and viral agents. Pathogen exclusion efforts are also costly and are addressed below in the biosecurity section. Numerous vaccinations not routinely used in conventional production are frequently utilised in ABF production with varying results. Streptococcus suis (S.suis), Haemophilus parasuis (Hps), Actinobacillus suis (A.suis), Actinobacillus pleuropneumoniae (App), Mycoplasma hyopneumoniae (M.hyo), Lawsonia intracellularis (ileitis), Erysipelas, Salmonella, Escherichia coli (E.coli), Porcine Reproductive and Respiratory Syndrome virus (PRRS), multivalent swine influenza (SIV), Porcine Circovirus Type 2 (PCV2) and other commercial or autogenous vaccines are frequently administered to a needle-wary pig. Unfortunately, most of these vaccines have variable efficacy and often are of marginal or reduced value in ABF pigs. Many of the vaccines available for use in the US produce little value in highchallenge conditions.
A 'closed herd' where replacement stocks are produced within the farm may have a stabilising health effect for many of the agents plaguing ABF production, but this strategy has not been an effective deterrent against PRRS, the most costly swine disease of US pigs (Neumann et al., 2005) and the most difficult to control. Other somewhat more effective methods of ABF health control include all-in, all-out pig flow by age group (rooms, houses, & sites), small population size, detergent wash and disinfection between groups, continuous sanitation, single sourced pigs, geographical isolation, delayed weaning age, frequent hand washing, and other sound husbandry practices. Overall, ABF production is much like flying an airplane; inherently safe but disastrous results may occur from even minor human errors or natural events.
Freedom from Specific Disease Agents
When pigs are free from Mycoplasma hyopneumoniae and PRRS virus, ABF production can compete on a cost of production basis with conventionally raised pigs. Feed conversion may be slightly depressed but growth rates, mortality and morbidity may be comparable to conventional production counterparts that are challenged with these agents. Thus, disease freedom is a logical opportunity to improve the lot of the ABF pig, avoiding excessive losses. Several methods of elimination of PRRS virus and M. hyo have evolved since the mid 1990s. Eradication methods are simple and generally cost-effective providing these agents can be excluded for periods longer than 12 months. Successful long-term ABF production requires freedom from PRRS virus, Mycoplasma hyopneumoniae, Actinobacillus pleuropneumoniae and as many of the eradicable agents as possible. Unfortunately, exclusionary biosecurity strategies have largely been impotent in geographical areas of the US with heavy pig density.
Biosecurity
Functional biosecurity is especially important in ABF systems. It may be divided into three significant areas of concern in all pig production systems: external biosecurity (bio-exclusion), internal biosecurity (bio-management) and agent containment (bio-containment). All are especially valuable in ABF production due to the lack of antibiotic interventions.
Bio-containment applies when a new agent enters a system or industry. Often a new agent enters a segment of production (i.e. boar stud) only to be transmitted to other sites because functional (effective) biosecurity methods are lacking. As discussed, internal biosecurity measures are those methods that reduce the impact of disease agents already present in the operation. This is accomplished by reducing the dose of the agent, increasing herd immunity to the agent, controlling the timing of infection and reducing environmental and biological stress. External biosecurity is designed to prevent a the introduction of a new agent, and individual agents often require specific interventions. Before designing exclusionary biosecurity strategies, the 'ecology' (complete life cycle) of a specific agent must be reasonably understood. Unfortunately, we often do not have full knowledge of these life cycles as is the case with PRRS virus. One last generality worth considering is that if a farm does not have security against sabotage or other crimes, then functional biosecurity is lacking. Uncontrolled entry of people is often responsible for introduction of new disease agents. ABF pigs are most susceptible to new agents, thus allowing entry of unwanted intruders leaves the population open to new disease introduction.
ABF biosecurity strategies should be developed utilising a Hazard Analysis Critical Control Point (HACCP) approach. Knowledge from scientifically applied field trial methodology, peer-reviewed publications and significant field experience should be heavily relied upon when establishing the critical control points. Extensive interviews and inputs from all farm staff should be included in the early stages of the hazard analysis assessments. Without participation of the farm employees, many critical control points (CCP) will be overlooked. Once the CCPs are identified, then and only then can intervention strategies be developed. Only those evidence-based intervention strategies that have demonstrable usefulness in the field are applicable. A hierarchy of interventions based on relative risk assessment can then be developed, in the end focusing on those factors which have the greatest impact and opportunity for success. A formula which is helpful in ranking appropriate biosecurity implementation decisions is as follows:

This formula facilitates analysis of each agent and each intervention strategy. These computations can then be used for choosing those strategies that have a final BIV greater than zero. Although arbitrary, the DD allows us to consider a customised score for the complexity of an intervention and the ability of the farm staff to adopt, implement and sustain an intervention procedure or process. It becomes farm- or system-specific, which is ideal in practice. If the risk of several diseases can be reduced by the same intervention, then the DEVs can be added together and the sum entered into the equation. As multiple agents are considered, the strength of the intervention strategy becomes apparent. Of course we do not know all the risk factors, values of disease exclusion or the percentage RR, but from the risk assessment tool, published information and biosecurity experts, one can arrive at reasonable approximations. Developing a value equation for each disease is often a matter of benchmarking diseased pigs with those that are disease-free in the same system. Some average disease cost numbers have been published and also useful benchmarks. The amount of RR for each biosecurity intervention and the perceived value for exclusion helps us arrive at a logical expectation for those interventions. With this approach only those interventions that have a value greater than zero are applied.
Calculating IC can be difficult and often relies on farm or industry experience. For example, the cost of building a shower facility is relatively straightforward but the variable costs associated with implementing showers for all who enter the farm are highly variable. Clothing costs, frequency of replacement, increased water use, shampoo, soap, washing machine, clothes dryer, added electrical usage, employee work time, morale, employee retention and many other details should be objectively calculated. It is useful to compare showering to exchange of boots and outerwear, but when working with ABF pigs best practices are needed. Downtime rules often create significant costs but have very limited exclusion value. Determining IC for downtime rules is difficult but no more difficult than the calculation of its DEV. Establishing universal DEV and IC for each economically important disease agent is worthy of considerable research dollars. Many of these issues must be solved before ABF production can compete in the US and global marketplace.
Conclusions
The livestock industry has a key responsibility to contribute meaningfully to producing enough food for the world's people while limiting resource use. The animals in production systems must be healthy in order to meaningfully meet that goal, and also to provide adequate well-being of the animals. Reduction or elimination of antibiotic use increases markedly the challenge of keeping animals healthy, and it demands attention to the entire herd health management programme.
The industry now has available a rich supply of feed ingredients that may improve animal health, and a few of them are reviewed here. Spray-dried plasma provides dramatic benefits, and immune egg products, acids, lactose, mannan oligosaccharide and others appear useful. The impact of dietary fibre is unclear.
Producing pigs without antibiotics clearly increases costs; US consumers have been willing to pay that additional cost for only certain cuts. Antibiotic-free (ABF) pigs are generally less healthy than conventionally produced pigs. The production of ABF pigs in the US has been dominated by large integrated production systems because they can provide the consistency of volume and quality needed. Producing pigs without antibiotics intensifies the need to manage pig health throughout a production pyramid. Vaccines and management strategies are useful but often inadequate. Elimination of PRRS and Mycoplasma hyopneumoniae is very useful when producing ABF pigs. Functional biosecurity is critical; a method for evaluating specific biosecurity interventions is described.
Overall, ABF production is much like flying an airplane; inherently safe but disastrous results may occur from even minor human errors or natural events.
References
Kaden, V., Steyer, H., Schnabel, J. and Bruer, W. 2005. Classical swine fever (CSF) in wild boar: the role of the transplacental infection in the perpetuation of CSF. In: J. Vet. Med. B Infect. Dis. Vet. Public Health, 52 (4), pp 161-164
Markestad, A. and Grave, K. 1997. Reduction in antibacterial drug use in Norwegian fish farming due to vaccinion. In: Gudding, R., Lillehaug, A., Midtlyng, P.J. and Brown, F. (eds.) In: Fish vacciniology. Basel: Kager, pp 365- 369
Michel, A., Venter, L., Espie, I.W. and Coetzee M.L. 2003. Mycobacterium tuberculosis in eight species at the national zoological gardens of South Africa, 1991–2001. In: Journal of Zoo and Wildlife Medicine; 34, pp 364–370
Mortensen, S. 1998. PRRS-infection through semen (Danish) In: Veterinärinformation, 2 pp. 3-9
Nielsen, N.C., Bille, N. and Svendsen, J. et al. 1976. Sygdomsbekempelse i svinebesaetninger (Control of diseases in swineherds). Kobenhavn: DSR Forlag ISBN: 87 7432 104 8
OIE, 2005.; Animal welfare: global issues, trends and challenges. In: Rev.sci.tech. Off.int.Epiz.Vol. 24 (2) ISSN 0253-1933
Slate, D., Rupprecht, C.E., Rooney, J.A., Donovan, D., Lein, D.H. and Chipman, R.B. 2005. Status of oral rabies vaccination in wild carnivores in the United States. In: Virus Res. Jul;111(1), pp 68-76
Stärk, K.D.C., Regula, G., Hernandez, J., Knopf, L., Fuchs, K., Morris, R.S. and Davies, P. 2006. Concepts for risk-based surveillance in the field of veterinary medicine and veterinary public health: Review of current approaches. In: BMC Health Services Research, 6-20 (doi:10.1186/1472-6963-6-20)
Söderlind, O., Olsson, E. and Smyth, C.J. et al. 1982. Effect of parenteral vaccination of dams on intestinal Escherichia coli in piglets with diarrhoea. In: Infect. Immun. 36 pp 900-906
Valle, P.S., Skjerve, E., Martin, S.W., Larssen, R.B., Osteras, O. and Nyberg, O. 2005. Ten years of bovine virus diarrhoea virus (BVDV) control in Norway: a cost-benefit analysis. In: Prev Vet Med. 72. pp 189-207
Von Rüden, S., Staubach, C., Kaden, K., Hess, R.G., Blicke, J., Kuehne, S., Sonnenburg, S., Froehlich, A., Teuffert, J. and Moennig, V. 2008. Retrospective analysis of the oral immunisation of wild boar populations against classical swine fever virus (CSFV) in region Eifel of Rhineland-Palatinate. In: Vet. Microbiol. 132 pp 29-38
Wallgren, P.T. 1994. The importance of diseases for daily growth of pigs. In: Proc.Nord Vet Congr.17 (2) pp. 106-110
Wathes, C.M., Miller, B.G., Bourne, F.J. 1989. Cold stress and postweaning diarrhoea in piglets inoculated orally or by aerosol. In: Anim. Prod., 49 pp 483-496
Wierup, M. 1983. The influence of antibiotics on the excreation of salmonella, Allmänt Veterinärmöte, Uppsala, Sveriges Veterinärförbund, Stockholm, pp 115-122
Wierup, M. 1999. The Swedish experience of limiting antimicrobial use. Proc. Argiculture´s role in managing antimicrobial resistance conference, Oct 24-26,Toronto, Canad , pp 99-109
Wierup, M. 2001. The Swedish experience of the 1986 year ban of antimicrobial Growth – promoters, with special reference to animal health, disease prevention, productivity and usage of antimicrobials. In: Microbial Drug Resistance, 7, 2 pp 183- 190
Wierup, M. and Wegener, H.C. 2006. Termination of AGP use and effect on subsequent production of broiler chickens in Sweden and Denmark during a 25 year period. In: Barug, D., de Jong, J., Kies, A.K. and Verstegen, M.W.A. Antimicrobial growth promoters, Where do we go from here? , Wageningen Academic Publishers, ISBN: 9076998876, www.wageningenacademic.com; pp 127-135
Wierup, M. 2008. The Swedish experiences of Salmonella control in food animals. In: International Seminar Nutztiere, BPT-Kongresse, Hannover 14 Nov 2008, Heraausgeber:bpt Akademie GmbH, Frankfurt am Main, www.bpt-akademie.de, pp. 17-24
Chapter 26
Hakanen, A., Jousimies-Somer, H., Siitonen, A., Huovinen, P., Kotilainen, P. 2003. Fluoroquinolone resistance in Campylobacter jejuni isolates in travelers returning to Finland: association of ciprofloxacin resistance to travel destination. In: Emerg.Infect.Dis. 9:267-270.
Roasto, M., Praakle, K., Korkeala, H., Elias, P. and Hänninen, M-L. 2007. Prevalence of Campylobacter in raw chicken meat of Estonian origin. In: Archiv für Lebensmittelhygiene 56, (49-72). 61-62.
Smith, J.L., Drum, D.J.V., Dai, Y., Kim, J.M., Sanchez, S., Maurer, J.J., Hofacre, C.L. and Lee, M.D. 2007. Impact of antimicrobial usage on antimicrobial resistance in commensal Escherichia coli strains colonizing broiler chickens. In: Applied Environmental Microbiology 73:1404-1414.
Teuber, M. 2004. Veterinary use and antibiotic resistance. In: Smulders, F.J.M. and Collins, J.D. (eds.) Food safety assurance and veterinary public health. Volume 2, Safety aasurance during food processing, Wageningen Academic Publishers, The Netherlands, pp. 229-241.
US Department of Health and Human Services http://www.hhs.gov/ news/press/2001pres/20010118b.html; accessed 24 May 2007
Van Looveren, M., Daube, G., De Zutter, L., Dumont, J-M., Lammens, C., Wijdooghe, M., Jouret, M., Cornelis, M. and Goossens, H. 2001. Antimicrobial susceptibilities of Campylobacter strains isolated from food animals in Belgium. In: Journal of Antimicrobial Chemotherapy, 48, 235-240.
Chapter 27
Baker, R. 2006 Health management with reduced antibiotic use – the U.S. experience. In: Anim. Biotec. 17:195-205.
Bosi, P., Han, I.K., Jung, H.J., Heo, K.N., Perini, S., Castellazzi, A.M., Casini, L., Creston, D. and Gremokolini, C. 2001. Effect of different spray dried plasmas on growth, ileal digestibility, nutrient deposition, immunity and health of early-weaned pigs challenged with E. coli K88. In: Asian-Aust. J. Anim. Sci. 14:1138-1143.
Chernysheva, L.V., Friendship, R.M., Gyles, C.L. and Dewey, C.E. 2003. Field trial assessment of the efficacy of specific egg-yolk antibody product for control of postweaning E. coli diarrhea. In: Veterinary Therapeutics 4:279-284.
Coffey, R.D., and Cromwell, G.L. 2001. Use of spray-dried animal plasma in diets for weanling pigs. In: Pig News and Information 22:39-48.
Duriez, P. and Topp, E. 2007. Temporal dynamics and impact of manure storage on antibiotic resistance patterns and population structure of Escherichia coli isolates from a commercial swine farm. In: App. Envir. Micro. 73:5486-5493.
Gilchrist, M.J., Greko, C., Wallinga, D.B., Beran, G.W., Riley, D.G. and Thorne, P.S. 2007. The potential role of concentrated animal feeding operations in infectious disease epidemics and antibiotic resistance. In: Enviro. Health Perspec. 115;313-316.
Hopwood, D.E., Pethick, D.W., Pluske, J.R. and Hampson, D.J. 2004. Addition of pearl barley to a rice-based diet for newly weaned piglets increases the viscosity of the intestinal contents, reduces starch digestibility and exacerbates post-weaning colibacillosis. In: Br. J. of Nutr. 92:419-427.
Kim, J.D., Hyun, Y., Sohn, K.S., Kim, T.J., Woo, H.J. and Wan, I.K. 2000. Effects of mannan oligosaccharide and protein levels on growth performance and immune status in pigs weaned at 21 days of age. In: J. Anim. Sci. Tech. 42:489-498.
Lusk, J.L., Norwood, F.B. and Pruitt, J.R. 2006. Consumer demand for a ban on antibiotic drug use in pork production. In: Amer. J. Agr. Econ. 88(4):1015-1033.
Macovei, L. and Zurek, L. 2007. Influx of Enterococci and associated antibiotic resistance and virulence genes from ready-to-eat food to the human digestive tract. In: App. Envir. Micro. 73:6740-6747.
Marquardt, R.R., Jin, L.Z., Kim, J-W., Fang, L., Frohlich, A.A. and Baidoo, S.K. 1999. Passive protective effect of egg-yolk antibodies against enterotoxigenic Escherichia coli K88+ infection in neonatal and early-weaned piglets. In: FEMS Immunology and Medical Microbiology 23:283-288.
McEwen, S., Taylor, D., Horzowski, A., Mitema, E., Angulo, F., Wierup, M., Li, D., Collignon, P., Pettigrew, J. and Bennett, R. 2003. Impacts of antimicrobial growth promoter termination in Denmark. The WHO international review panel's evaluation of the termination of the use of antimicrobial growth promoters in Denmark. World Health Organization. McMahon, M.A.S., Xu, J., Moore, J.E., Blair, I.S. and McDowell, D.A. 2007. Environmental stress and antibiotic resistance in food-related pathogens. In: App. Envir. Micro. 73:211-217.
Miguel, J.C., Laski, P.J. and Pettigrew, J.E. 2006. Efficacy of a mannan oligosaccharide and antimicrobial on the gastrointestinal microbiota of young pigs. In: J. Anim. Sci. 84 (Suppl. 1):44.
Miguel, J. C., Rodriguez-Zas, S.L. and Pettigrew, J.E. 2004. Efficacy of Bio-Mos for improving nursery pig performance. In: J. Swine Health Prod. 12:296-307.
Neumann, E.J., Kliebenstein, J.B., Johnson, C.D., Mabry, J.W., Bush, EJ., Seitzinger, A.H., Green, A.L. and Zimmerman, J.J. 2005. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. In: J. Am. Vet. Med. Assoc. 227:385-392.
Nollet, H., Deprez, P., Van Driessche, E. and Muylle, E. 1999. Protection of just weaned pigs against infection with F18+ Escherichia coli by non-immune plasma powder. In: Veterinary Microbiology 65:37-45.
Owusu-Asiedu, A., Nyachoti, C.M., Baidoo, S.K., Marquardt, R.R. and Yang, X. 2003. Response of early-weaned pigs to an enterotoxigenic Escherichia coli (K88) challenge when fed diets containing spraydried porcine plasma or pea protein isolate plus egg yolk antibody. In: J. Anim. Sci. 81:1781-1789.
Palacios, M.F., Flickinger, E.A., Grieshop, C.M., Collier, C.T. and Pettigrew, J.E. 2004. Effects of lactic acid and lactose on the digestive tract of nursery pigs. In: J. Anim. Sci. 82:(Suppl. 1):139 (Abstr.).
Partanen, K. 2001. Organic acids-their efficacy and modes of action in pigs. In: Gut Environment of Pigs; Piva, A.; Bach Knudsen, K. E.; Lindenburg, J. E., Eds.; Nottingham University Press.
Phillips, I., Casewell, M., Cox, T., DeGroot, B., Friis, C., Jones, R., Nightingale, C., Preston, R. and Waddell, J. 2004. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. In: J. Antimicrobial Chemoth. 53:28-53.
Pierce, J.L., Cromwell, G.L., Lindemann, M.D., Russell, L.E. and Weaver, E.M. 2005. Effects of spray-dried animal plasma and immunoglobulins on performance of early weaned pigs. In: J Anim. Sci. 83:2876-2885.
Shashidhara, R.G. and Devegowda, B. 2003. Effect of dietary mannan oligosaccharide on broiler breeder production traits and immunity. In: Poult. Sci. 82:1319-1325.
Sørum, M., Johnsen, P.J., Aasnes, B., Rosvoll, T., Kruse, H., Sundsfjord, A. and Simonsen, G.S. 2006. Prevalence, persistence, and molecular characterization of glycopeptides-resistant Enterococci in Norwegian poultry and poultry farmers 3 to 8 years after the ban on avoparcin. In: App. Envir. Micro. 72:516-521.
Tokach, M.D., Nelssen, J.L. and Allee, G.L. 1989. Effect of protein and(or) carbohydrate fractions of dried whey on performance and nutrient digestibility of early weaned pigs. In: J. Anim. Sci. 67:1307- 1312.
Touchette, K.J., Carroll, J.A., Allee, G.L., Matteri, R.L., Dyer, C.J., Beausang, L.A. and Zannelli, M.E. 2002. Effect of spray-dried plasma and lipopolysaccharide exposure on weaned pigs: I. Effects on the immune axis of weaned pigs. In: J. Anim. Sci. 80:494-501.
van Dijk, A.J., Everts, H., Nabuurs, M.J.A., Margry, R.J.C.F. and Beynen, A.C. 2001. Growth performance of weanling pigs fed spray-dried animal plasma: a review. In: Livestock Production Science 68:263-274.
Wenk, C. 2001. The role of dietary fibre in the digestive physiology of the pig. In: Animal Feed Sci. Tech. 90:21-33.
Yokoyama, H., Hashi, T., Umeda, K., Icatlo Jr, C., Kuroki, M., Ikemore, Y. and Kodama, Y. 1997. Effect of oral egg antibody in experimental F18+ Escherichia coli infection in weaned pigs. In: J. Vet. Med. Sci. 59:917-921.
Zhang, R., Effleston, K., Rotimi, V. and Zeckhauser, R.J. 2006. Antibiotic resistance as a global threat: Evidence from China, Kuwait and the United States. In: Globalization and Health 2:1-14.
Chapter 28
AFSSA. Rapport Intermediaire. Utilisation des Antibiotiques chez l' animal et Re´sistance aux Antibiotiques chez les Bacte´ries d' origine Animal. Agence Francaise de Se´curite Sanitaire des Aliments. 2003. http://www.afssa.fr
Bengtsson, B. and Wierup, M. 2006. Antimicrobial resistance in bacteria from food animals in Scandinavia after termination of antimicrobials for growth promotion. In: Animal Biotechnology. 2006;17(2):147-56.
This article (Chapter 27, Case Study in USA) belongs to the Prevention of Infectious Diseases in Livestock and Wildlife publication. www.balticuniv.uu.se. Engormix thanks the author and publisher for this contribution.
Related topics
Authors:
University of Illinois
Recommend
Comment
Share
Berge Veterinary Consulting
21 de marzo de 2014
Thank you for a nice review article of the alternative nutritional solutions to antibiotics. Furthermore, I appreciate that you clearly state the importance of biosecurity and management measures, as people are always seeking quick alternative nutritional solutions for a very complex problem.
Recommend
Reply
Recommend
Reply
1 de agosto de 2019
Beste Anna, Dear Anna,
We met a few times at AMCRA meetings where i represent UPV ( the french Speaking Belgian Vets).I am also founder of an association of veterinary consultants AVC
http://www.avc-eu.de
I think that you could be a good member of our association. Please do not hesitate to contact the secretary and you can make reference to me.
BR, MVG
Bill
Dr Bill VANDAELE
Dr Vet Med-Lic Zoot-E.P.A-MAVC
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.









