From follicle pool to piglet birth weight (variation)
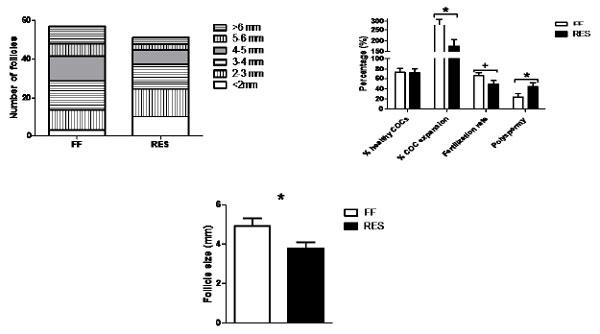
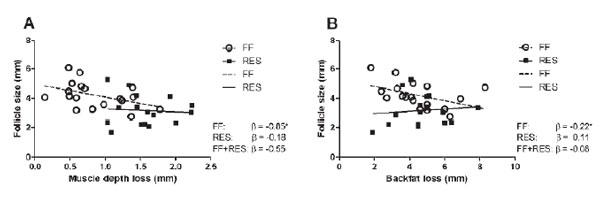
![Effect of average CL diameter measured using transrectal ultrasound (TUS) at ~ day 30 of pregnancy [5.5 to 7.8 mm (n = 23); 7.9 to 8.9 mm (n = 47); and 9.0 to 10.5 mm (n = 30)] on BW of total piglets born [P = 0.04; corrected for litter size class P < 0.0001] (panel A) standard deviation (SD) of BW of the total piglets born (P = 0.02) (panel B). ab P< 0.05. LSM±SE (from Da Silva et al., 2017b).](/_next/image/?url=https%3A%2F%2Fimages.engormix.com%2FS_articles%2F0_569_483.jpg&w=828&q=75)
Agrovision BV. Kengetallen spiegel zeugen. Agrovision BV, Deventer, The Netherlands, 2016.
Baxter EM, Rutherford KMD, D'Eath RB, Arnott G Turner SP, Sandøe P, Moustsen VA, Thorup F, Edwards SA,Lawrence AB. The welfare implications of large litter size in the domestic pig II: Management factors. Animal Welfare, v.22, p.219-238, 2013.
Beaulieu AD, Aalhus JL, Williams NH, Patience JF. Impact of piglet birth weight, birth order, and litter size on subsequent growth performance, carcass quality, muscle composition, and eating quality of pork. Journal of Animal Science, v.88, p.2767-2778, 2010.
Calus MPL. Genomic breeding value prediction: Methods and procedures. Animal, v.4, p.157-164, 2010.
Clowes EJ, Aherne FX, Foxcroft GR, Baracos VE. Selective protein loss in lactating sows is associated with reduced litter growth and ovarian function. Journal of Animal Science, v.81, p.753-764, 2003.
Costermans NGJ. 2020: Physiological and molecular aspects of ovarian follicular developmental competence in sows. PhD, Wageningen University, Wageningen.
Costermans NGJ, Keijer J, Van Schothorst EM, Kemp B, Keshtkar S, Bunschoten A, Soede NM, Teerds KJ In ovaries with high or low variation in follicle size, granulosa cells of antral follicles exhibit distinct size-related processes. Molecular Human Reproduction, v.25, p.614-624, 2019a.
Costermans NGJ, Soede NM, Middelkoop A, Laurenssen B, Koopmanschap RE, Zak L, Knol EF, Keijer J, Teerds KJ, Kemp B. Influence of the metabolic state during lactation on milk production in modern sows. Animal, (in press).
Costermans NGJ Soede NM Tricht F Blokland M Kemp B Keijer J Teerds KJ. Follicular fluid steroid profile in sows: relationship to follicle size and oocyte quality. Biology of Reproduction, 1-11 p, doi:10.1093/biolre/ioz175, 2019b.
Costermans NGJ, Teerds KJ, Keijer J, Knol EF, Koopmanschap RE, Kemp B, Soede NM. Follicular development of sows at weaning in relation to estimated breeding value for within-litter variation in piglet birth weight. Animal, v.13, p.554-563, 2019c.
Costermans NGJ, Teerds KJ, Middelkoop A, Roelen BAJ, Schoevers EJ, Van Tol HTA, Laurenssen B, Koopmanschap RE, Zhao Y, Blokland M, Van Tricht F, Zak L, Keijer J, Kemp B, Soede NM. Consequences of negative energy balance on follicular development and oocyte quality in primiparous sows. Biology of Reproduction, 1- 11 p, doi:10.1093/biolre/ioz175, 2019d
Da Silva CLA. Relations between ovarian & embryonic traits in pigs: effects of genetic selection for litter traits at birth. PhD thesis, Wageningen University, Wageningen, 2018.
Da Silva CLA, Broekhuijse MLWJ, Laurenssen BFA, Mulder HA, Knol EF, Kemp B, Soede NM. Relationship between ovulation rate and embryonic characteristics in gilts at 35 d of pregnancy. Journal of Animal Science, v.95, p.3160-3172, 2017a.
Da Silva CLA, Laurenssen BFA, Knol EF, Kemp B, Soede NM. Validation of transrectal ultrasonography for assessment of corpora lutea characteristics in pregnant sows and its relationship with litter characteristics at birth. Translational Animal Science, v.1, p.507-517, 2017b.
Da Silva CLA, Mulder HA, Broekhuijse M, Kemp B, Soede NM, Knol EF. Relationship Between the Estimated Breeding Values for Litter Traits at Birth and Ovarian and Embryonic Traits and Their Additive Genetic Variance in Gilts at 35 Days of Pregnancy. Frontiers in Genetics, v.9, article 111, 1-11 p., 2018.
Da Silva CLA, Van Den Brand H, Laurenssen BFA, Broekhuijse MLWJ, Knol EF, Kemp B, Soede NM. Relationships between ovulation rate and embryonic and placental characteristics in multiparous sows at 35 days of pregnancy. Animal, v.10, p.1192-1199, 2016.
Damgaard LH, Rydhmer L, Løvendahl P, Grandinson K. Genetic parameters for within-litter variation in piglet birth weight and change in within-litter variation during suckling. Journal of Animal Science, v.81, p.604-610, 2003.
Freking BA, Lents CA, Vallet JL. Selection for uterine capacity improves lifetime productivity of sows. Animal Reproduction Science, v.167, p.16-21, 2016.
Foxcroft GR, Dixon WT, Novak S, Putman CT, Town SC, Vinsky DA. The biological basis for prenatal programming of postnatal performance in pigs. Journal of Animal Science, v.84 (E Supplement), p.E1-5-E112, 2014.
Hunter MG, Wiesak T. Evidence for and implications of follicular heterogeneity in pigs. Journal of Reproduction and Fertility, Supplement v.40, p.163-177, 1990.
Kemp B, Da Silva CLA, Soede NM. Recent advances in pig reproduction: Focus on impact of genetic selection for female fertility. Reproduction in Domestic Animals, v.53, p.28-36, 2018.
Kemp B, Soede NM. Consequences of variation in interval from insemination to ovulation on fertilization in pigs. Journal of Reproduction and Fertility, Supplement 52, p.79-89, 1997.
Knox RV. 2005: Recruitment and selection of ovarian follicles for determination of ovulation rate in the pig. Domestic Animal Endocrinology, v.29, p.385-397.
Marchal R, Vigneron C, Perreau C, Bali-Papp A, Mermillod P. Effect of follicular size on meiotic and developmental competence of porcine oocytes. Theriogenology, v.57, p.1523-1532, 2002.
Milligan BN, Fraser D, Kramer DL. Within-litter birth weight variation in the domestic pig and its relation to preweaning survival, weight gain, and variation in weaning weights. Livestock Production Science, v.76, p.181-191, 2002.
Père MC, Dourmad JY, Etienne M. Effect of Number of Pig Embryos in the Uterus on Their Survival and Development and on Maternal Metabolism. Journal of Animal Science, v.75, p.1337-1342, 1997.
Pope WF, Xie S, Broermann DM, Nephew KP. Causes and consequences of early embryonic diversity in pigs. J Reprod Fert Suppl, v.40, p.251-260, 1990.
Prunier A, Soede N, Quesnel H, Kemp B. Productivity and longevity of weaned sows In: J.R. Pluske; J. de Lividich; M.W.A. Verstegen, (eds), Weaning the pig. Wageningen Academic Publishers, Wageningen, p.385-419, 2003.
Quesnel H, Brossard L, Valancogne A, Quiniou N. Influence of some sow characteristics on within-litter variation of piglet birth weight. Animal, v.2, p.1842-1849, 2008.
Quesnel H, Farmer C, Devillers N. Colostrum intake: Influence on piglet performance and factors of variation. v.146, p.105-114, 2012.
Rehfeldt C, Tuchscherer A, Hartung M, Kuhn G. A second look at the influence of birth weight on carcass and meat quality in pigs. Meat Science, v.78, p.170-175, 2008.
Rutherford KMD, Baxter EM, D'Eath RB, Turner SP, Arnott G, Roehe R, Ask B, Sandøe P, Moustsen VA, Thorup F, Edwards SA, Berg P, Lawrence AB. The welfare implications of large litter size in the domestic pig I: biological factors. Animal Welfare, v.22, p.199-218, 2013.
Van Den Brand H, Soede NM, Kemp B. Supplementation of dextrose to the diet during the weaning to estrus interval affects subsequent variation in within-litter piglet birth weight. Animal Reproduction Science, v.91, p.353-358, 2006.
Van Den Brand H, Van Enckevort LCM, Van Der Hoeven EM, Kemp B. Effects of dextrose plus lactose in the sows diet on subsequent reproductive performance and within litter birth weight variation. Reproduction in Domestic Animals, v.44, p.884-888, 2009.
Wientjes J, Soede NM, Van Den Brand H, Kemp B. Nutritionally induced relationships between insulin levels during the weaning-to-ovulation interval and reproductive characteristics in multiparous sows: II. Luteal development, progesterone and conceptus development and uniformity. Reproduction in Domestic Animals, v.47, p.62-68, 2012a.
Wientjes JGM Soede NM Van Der Peet-Schwering CMC Van Den Brand H Kemp B. Piglet uniformity and mortality in large organic litters: Effects of parity and pre-mating diet composition. Livestock Science, v.144, p.218- 229, 2012b.
Wientjes JGM, Soede NM, Knol EF, Van Den Brand H, Kemp B. Piglet birth weight and litter uniformity: Effects of weaning-to-pregnancy interval and body condition changes in sows of different parities and crossbred lines. Journal of Animal Science, v.91, p.2099-2107, 2013.
Xie S, Broermann DM, Nephew KP, Geisert RD, Pope WF. Ovulation and early embryogenesis in swine. Biology of Reproduction, v.43, p.236-240, 1990.
Zak LJ, Gaustad AH, Bolarin A, Broekhuijse MLWJ, Walling, GA Knol EF. Genetic control of complex traits, with a focus on reproduction in pigs. Molecular Reproduction and Development, v.84, p.1004-1011, 2017.







