Effects of dietary supplementation with mannan oligosaccharides on performance of commercial sows and their litters
International consumer groups and legislators are demanding the reduction in use of antimicrobial growth promoters in livestock feeds. It is thought that the use of antibiotics in animal feed increases the number of antibiotic resistant bacteria in the animal and the environment and makes human diseases more difficult to treat (Corpet, 1988; Langlois et al., 1978 a,b). Because of these concerns and legislation against sub-therapeutic antibiotic use in livestock, producers are looking for alternative means of bringing about similar performance, health and economic benefits. Options being explored by a number of researchers include genetic selection for more disease-resistant animals, alternative feed additives that may have growth promoting properties and improving the immune status of the animal.
Mannan oligosaccharides are complex carbohydrates derived from the cell wall of yeast with mannose as the primary carbohydrate. Mannan oligosaccharide (MOS) has been shown in a number of livestock species to provide benefits similar to antibiotic growth promoters (Sims et al., 1998; Maxwell, 1999). In addition, immunoglobulin status of chickens has been shown to be greater in birds receiving MOS (Savage et al., 1996). In pigs, MOS supplementation has resulted in better gains, feed conversion, enhanced lymphocyte transformation and immunoglobulin concentrations than in unsupplemented animals (Brendemuhl and Harvey, 1999; Spring and Pirvulescu, 1998; Van der Beke, 1997). In addition, field data suggest that piglets from sows supplemented with MOS are more uniform in weight and have better survival to weaning than piglets from unsupplemented sows (Newman, 2001).
The demonstrated effect of MOS on immunoglobulins (Ig) in poultry and piglets implies that more immunoglobulins could be available in the colostrum of sows supplemented with MOS. Colostrum represents the accumulated secreted antibodies of the mammary gland. In pigs, placentation is epithelichorial, which means that the fetal chorionic epithelium is in contact with intact uterine epithelium. In animals with this type of placenta, transplacental passage of immunoglobulin molecules is totally prevented. This makes the newborn piglet entirely dependent on antibodies received in the colostrum. The sow colostrum is rich in IgG and IgA, but levels of IgG drop rapidly and IgA predominates in the milk as lactation proceeds (Curtis and Bourne, 1970). In the pig, protein absorption seems to be selective with IgG and IgM preferentially absorbed. Improving the overall immunoglobulin concentration of colostrum may provide benefits to the young piglet in two ways.
First, the piglet receives higher concentrations of protective immunoglobulins, which increases resistance to infection during the period of time required for this animal to develop its own immune defenses. The piglet acquires this higher level of immunoglobulins in the same volume of colostrum, thus making it more immunocompetent than a piglet receiving less passive transfer. Secondly, immunoglobulins are proteins; and therefore enhancing these levels may provide nutritional benefits to the growing pig.
Materials and methods
This experiment was conducted at a commercial sow farm in North Carolina utilizing PIC genetics.
Gestating and lactating sows were individually penned. All diets were nutritionally adequate (NRC, 1998) and were based on corn, soybean meal and cookie meal. Experimental diets were created by substituting 4 lbs of Bio-Mos® for corn in the gestation diets and 2 lbs/ton in the lactation diets.
Diet samples were taken weekly, and a composite sample was analyzed for mycotoxin levels.
Experimental diets contained a synthetic red iron oxide as a coloring agent to verify correct delivery of feeds from the mill to the farm. Gestating sows were fed based on sow body condition once daily by an automated feed drop system; and lactating sows were hand-fed three times daily.
Sows were individually weighed when moved from the gestation barn to the farrowing house at day 112 of gestation and again at weaning (day 21).
Individual litters were weighed at processing (30 hrs after birth) and at weaning. Pre-weaning mortality was recorded on a daily basis, and any sow deaths were also recorded. All pig transfers were done by the time of litter processing (iron shots, castration, clipping of needle teeth and tail docking), and transfers were only within dietary treatment groups. Days post-weaning for sows to return to estrus were also monitored with the Insight® data management program. Feed deliveries were recorded at the feed mill, but a determination of sow average daily feed intake was not made. All buildings were mechanically ventilated to maintain a thermoneutral environment for the sows. Zonal heating for the pigs was provided by heat lamps.
This experiment was conducted during the late summer months of 2000 through early January 2001.
Pre-nursing colostrum samples were taken from sows and analyzed as blind samples by Venture Laboratories, Lexington, KY. The samples were analyzed for IgA, IgG and IgG. Exogenous oxytocin was not used in the collection of the colostrum samples.
All data were analyzed by means of paired t-tests assuming equal variance between treatment means.
Results and discussion
Dietary mycotoxin concentrations (Table 1) were similar between diets with the exception of aflatoxin (27.30 and 45.03 ppb for the Bio-Mos® and control treatments, respectively). It is possible that Bio- Mos® may have bound some aflatoxin and rendered it unabsorbable. The effects of Bio-Mos® on aflatoxin in feed is currently under investigation. The reductions in aflatoxin levels could be a partial reason for the improvements observed in sow and litter performance in the present study.
Table 1. Pooled dietary mycotoxin analyses.
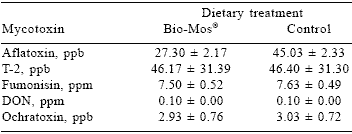
Initial and final sow weights between the two groups were similar (Table 2). The number of pigs born live and stillborns were also similar between dietary groups. Average birth weight (P<0.05) and weaning weight (P<0.01) were increased by addition of Bio-Mos® addition to the sow diet; consequently litter weight gain and average daily gain (ADG) of the pigs were also increased in this treatment group. Pre-weaning mortality was decreased from 11.27 to 9.09% (19.30% reduction) by supplementing sow diets with Bio-Mos® (P<0.01). Sows given Bio- Mos®-supplemented diets returned to estrus more quickly (5.20 vs 7.27 days; P<0.01). Furthermore, 88.02% of the MOS-supplemented sows returned to estrus, while only 77.76% of the control sows were returned to the breeding herd. This could have been a function of culling procedures or of sow parity. However, average sow parity was nearly identical between dietary groups (3.23 vs 3.29), and the individual distribution of parity within dietary groups (Figure 1) did not display any wide fluctuations.
Table 2. Performance summary for sows fed Bio-Mos® in a commercial production system
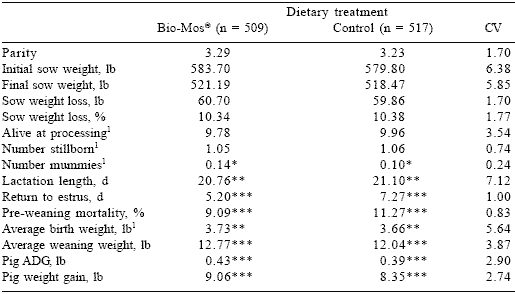
1Recorded at processing (~30 h post-farrowing).
*(P< 0.10)
**(P< 0.05.)
***(P< 0.01)
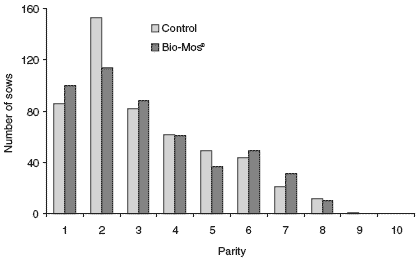
Figure 1.Distribution of parity by dietary treatment.
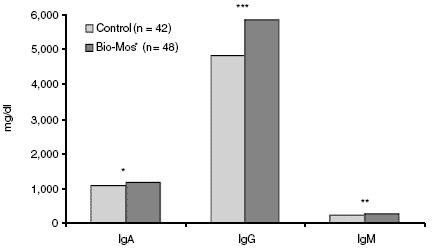
Figure 2.Effect of Bio-Mos® on pre-nursing sow colostrum immunoglobulin content (*P<0.10; **P<0.05%, ***P<0.01).
The improvements in litter weight gain and preweaning mortality may be explained through the alterations in the colostrum immunoglobulin profiles. Concentrations of IgA, IgG and IgM were all increased by the addition of Bio-Mos® to the sow diets (Figure 2). Thus, the pigs from sows receiving Bio-Mos® could be expected to be healthier (decreased pre-weaning mortality) and grow faster because of the enhancements to the colostrum. The modes of action for Bio-Mos®, as outlined by Pettigrew (2000), would seem to apply in this study. Bio-Mos® may have bound aflatoxin, which would be expected to enhance sow and litter performance. Additionally, Bio-Mos® enhanced the immune response as evidenced by altered colostrum immunoglobulin profiles. Conclusions Dietary supplementation with Bio-Mos® improves several variables of sow and litter productivity. While the economics of productivity variables differ among farms and markets, based on criteria such as increased piglet performance, improved sow reproductive status and reduced non-productive sow days, the use of Bio-Mos® should be economically advantageous.
References
Author: D.W. FUNDERBURKEBrendemuhl, J.H. and M.R. Harvey. 1999. An evaluation of mannan oligosaccharide for pigs: performance response during nursery and growing-finishing phases. Poster presented at Alltech’s 15th Annual Symposium, Lexington, Kentucky, April.
Corpet, D.E.1988. Antibiotic resistance from food. N. Eng. J. Med.318:1206-1207.
Curtis, J. and F.J. Bourne. 1970. Immunoglobulin quantitation in sow serum, colostrum, and milk and the serum of young pigs. Biochem. Biophys. Acta. 236:319-325.
Langlois, B.E., G.L. Cromwell and V.W. Hays. 1978a. Influence of chlorotetracycline in swine feed on reproductive-performance and on incidence and persistence of antibiotic resistant enteric bacteria. J. Anim. Sci. 46(5):1369-1382.
Langlois, B.E., G.L. Cromwell and V.W. Hays. 1978b. Influence of type of antibiotic and length of antibiotic feeding period on performance and persistence of antibiotic resistance. J. Anim. Sci. 46(5):1383-1396.
Maxwell, C. 1999. Nutrition and management of the early-weaned pig. In: Biotechnology in the Feed Industry: Proceedings of Alltech’s 15th Annual Symposium (T.P. Lyons and K.A. Jacques, eds), Nottingham Press University, Nottingham, UK, pp. 203-222.
Newman, K. 2001. Mannanoligosaccharides: natural polymers with significant impact on the gastrointestinal microflora and the immune system. In: Biotechnology in the Feed Industry. Proceedings of Alltech’s 17th Annual Symposium (T.P. Lyons and K.A. Jacques, eds), Nottingham University Press, Nottingham, UK, pp. 167-174.
NRC, 1998. Nutrient Requirement of Swine (10th Ed.). National Academy Press. Washington, DC. Pettigrew, J.E. 2000. Bio-Mos effects on pig performance: a review. In: Biotechnology in the Feed Industry, Proceedings of the 16th Annual Symposium (T.P..Lyons and K.A. Jacques, eds) Nottingham University Press, UK. pp. 31-34.
Savage, T.F., P.F. Cotter, and E.I. Zakrzewska. 1996. The effect of feeding mannan oligosaccharide on immunoglobulins, plasma IgG and bile IgA of Wrolstad MW male turkeys. Poultry Sci. 75(suppl. 1):143.
Sims, M.D., P. Spring, and A.E. Sefton. 1998. Effect of mannan oligosaccharide on performance of commercial broiler chickens. Poultry Sci. 77(suppl. 1):89.
Spring, P. and M. Pirvulescu. 1998. Mannan oligosaccharide: its logical role as a natural feed additive for piglets. In: Biotechnology in the Feed Industry: Proceedings of Alltech’s 14th Annual Symposium (T.P. Lyons and K.A. Jacques, eds), Nottingham Press University, Nottingham, UK, pp. 553-561.
Van der Beke. 1997. Het gebruik van mannan oligosacchariden (Bio-Mos®) en lactobacillen (Lacto-Sacc) inbiggenvoeders. Thesis. Highschool Gent, Department Biotechnological Sciences, Landscape Management and Agriculture. Gent, Belgium.
Cape Fear Consulting, LLC, Warsaw, NC, USA






.jpg&w=3840&q=75)