Dietary organic and inorganic selenium for hyperovulatory firstparity sows: antioxidant status, hormonal response, embryo development and reproductive performance
Although it has been known for many years that an increase in ovulation rate in pigs is detrimental to embryo survival, the precise mechanisms involved are not well understood. The poor quality of the oocytes shed by these supplementary mature follicles has been evoked along with its consequences on either fertilisation of ova and/or the ability of the embryo cells for development and differentiation (Driancourt et al., 1998).
The focus that has been put on hyperprolificacy during the last 10 to 20 years has induced heterogeneity of piglet weight within the litter (Tribout et al., 2003; Le Cozler et al., 2004). A lack of uniformity in piglet weights has carry-over effects after birth in terms of growth performance and quality of carcass and meat (Gondret et al., 2005). Several post-natal strategies (adoption, split-weaning) have been proposed to decrease within-litter variation of piglet weights, but with limited success (Matte et al., 1991). A prenatal strategy appears essential, but a reliable improvement in this production trait requires a better understanding of its in utero physiological causes.
Ovulation and antioxidation
Some work carried out in other polytocous species can help to identify the ovarian metabolic perturbations inducing such an impairment of oocyte quality. The ovarian metabolism under hormonal regulation is very active and is subjected to drastic and short-term changes during the reproductive life of females. This intense metabolism generates reactive oxygen species (ROS) and free radicals (FR) that are potentially toxic and damaging to ovarian tissue.
Those toxic metabolites must be neutralized locally in order to maintain tissue integrity and function. The protection against ROS and FR is brought by reactions with antioxidants such as the enzymatic degradation of various peroxides by selenium (Se)-dependent glutathione peroxidase (GSH-Px). The glutathione (GSH) is a co-factor for the Se-GSH-Px that allows the reduction of toxic peroxides (ROOH). Selenium, in the form of selenocysteine, is an important and essential element of GSH protein (McDowell, 1992). Two vitamins, E and C, are also well known for their antioxidant properties. Lipid soluble vitamin E is known as a non-enzymatic antioxidant that neutralizes ROS and FR while GSH-Px is an enzymatic component of the antioxidant system. It is generally considered that, nutritionally, a ‘sparing effect’ exists between vitamin E and selenium (through Se-GSH-Px antioxidant activities) for total antioxidant capacity of the animal (McDowell, 2000; LeGrusse et Watier, 1993). In fact, both GSH-Px and vitamin C, in addition to their direct antioxidant roles, act in synergy with vitamin E to recycle (or regenerate) tocopheryl quinone (the oxidized form of vitamin E) to tocopherol (the bioactive form of vitamin E) (LeGrusse et Watier, 1993).
Vitamins E and C as well as GSH are present in the ovarian tissue of the rat in amounts that vary according to the stage of the estrous cycle (Aten et al., 1992; 1994). Vitamin C and selenium are considered to be important factors for the stability and integrity of the growing follicle. In fact, the in vitro survival of follicles is considerably increased by the presence of either vitamin C alone (from 33 to 88 %, Murray et al., 2001) or both vitamin C and selenium in the media culture (from 29 to 87 %, Rose et al., 1999).
In vivo effects of antioxidants on ovaries
In beef cattle (Segerson et al., 1977) and sheep (Segerson and Ganapathy, 1980), parenteral administration of vitamin E and selenium maximized fertilization rate of ova as compared to unsupplemented controls. In mice, dietary supplements of both vitamins C and E delayed the age-related decrease in ovulation rate, probably through a decrease in follicular atresia (Tarìn et al., 1998). In this last case, the potential generation of oxidative stress induced by age on oocytes and granulosa cells would be prevented by the antioxidant vitamins. However, in the pig, little information is available on the importance of adequate antioxidant reserves (or status) for optimal ovulation both in terms of quantity and quality. Nevertheless, it has been recently shown that there is a considerable accumulation of vitamin C (100-fold higher than the circulation levels) in the functional corpora lutea (Petroff et al., 1998). It was also suggested that periods of maximal luteal and follicular activities are associated with increased concentrations of ascorbate within the tissue (Petroff et al., 1997). However, it is not clear if this ascorbate accumulation is related to the role of vitamin C for collagen synthesis or as antioxidant.
In the present experiment, we have hypothesized that there might be a transient lack of metabolically available antioxidant during the pre-ovulatory phase of the estrous cycle in sows, which could lead to a suboptimal quality of ovulation and eventually of embryos. In this way, metabolically available antioxidant from dietary selenium during the pre-ovulatory phase of the estrous cycle may be beneficial for the quality of ovulation and for the uniformity of embryo weights within litters. We have focused on the importance of the dietary forms (inorganic vs organic) of selenium on the antioxidant status during the estrous cycle and early pregnancy along with uniformity of conceptus development at 30 days of gestation (overall litter mean and coefficient of variation within the litter). This last criterion was used as an indication of the quality of ovulation and fertilization of ova and subsequently of the ability of the embryo cells for development and differentiation. The source of selenium may be of importance since organic selenium (predominantly yeast selenomethionine) is recognized as more bioavailable than inorganic selenium sources such as selenite. Moreover, with organic selenium this higher provision of selenium can be achieved without any risk of toxicity.
Experimental design
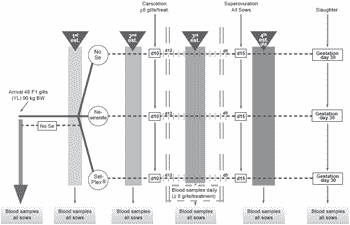
This project aimed to determine the effect of dietary selenium as inorganic sodium selenite or organic selenium (Sel-Plex®, Alltech Inc.) on antioxidant status and hormonal profile after puberty and in early gestation of gilts as well as on maternal selenium transfer to embryos, embryonic antioxidant status (Ferric Reducing Antioxidant Power (FRAP)), GSH-Px activity and litter performance. Three groups of 16 gilts each received one of the three dietary treatments, starting at first pubertal estrus and lasting through 30 days post-AI (AI at the fourth estrus): control basal diet, without supplementation (Se content of 0.203 ppm) (Table 1); selenite, basal diet + 0.3 ppm Se as sodium selenite; Sel-Plex®, basal diet + 0.3 ppm as Sel-Plex®. Blood was collected from all gilts on the day after each estrus and on day 30 post-AI. Blood was also collected daily from day –4 until day +4 of the third estrus (day 0) in 8 Control, 9 selenite and 8 Sel-Plex® canulated gilts (Figure 1).
Table 1. Composition of the basal experimental dieta.

aThe Se content of the basal diet was 0.203 ppm
bProvided per kg of diet: Mn as manganous oxide, 30 mg; Zn as zinc oxide, 100 mg; Fe as ferrous sulfate, 100 mg; Cu as copper sulfate, 25 mg; and I as calcium iodate, 300 μg.
cProvided per kg of diet: vitamin A, 10,000 IU; vitamin D3, 2,000 IU; vitamin E, 44 IU; menadione, 2.2 mg; thiamin, 2 mg; riboflavin, 5 mg; niacin, 25 mg; pantothenic acid, 16 mg; pyridoxine, 3 mg; folic acid, 10 mg; biotin, 250 μg; vitamin B12, 20 μg, and choline, 300 mg.
To enlarge the image, click here
Figure 1. Experimental design and schedule.
Long-term effects on selenium-related metabolites At the end of the experimental period, day 30 of gestation, blood selenium was lower (P<0.01) by 18% in Control than in Se-supplemented groups and higher (P<0.01) by 16% in Sel-Plex® than in selenite sows (Figure 2). The amplitude of the present responses using whole blood selenium was different than that reported by Mahan and Kim (1996). Their corresponding control vs Se-supplemented and organic vs inorganic selenium effects using serum selenium as an indicator at 35 days of gestation was more and less pronounced, respectively, than in the present experiment. Whole blood selenium was chosen as indicator of circulating selenium status in the present experiment because the red blood cells are an important selenium pool with approximately 50% of the overall selenium in circulation (Matte and Giguère, unpublished observations).
Blood Se-GSH-Px tended to be lower (P<0.06) in control than in Se-supplemented groups but only from the third estrus. From the time of mating (AI), GSHPx in animals given selenite was higher (P<0.01) than in those given Sel-Plex® (Figure 3). Such an effect can be linked to those reported recently by Mahan and Peters (2004), where GSH-Px in serum over four parities was numerically higher by approximately 6% in selenitecompared to Sel-Plex®-fed sows at 30 days of gestation. The values here were 8.5% higher. This difference may be related to the fact that the phosphate form of selenium, which is essential for the gene transcription for Se-GSH-Px synthesis, would be more readily available from selenite than from selenomethionine (Flohé et al., 1997).
No effect (P<0.19) was noted on ferric reducing antioxidant power (FRAP) in circulation, an indicator of the overall total antioxidant activity within the body. Plasma triiodothyronine (T3), a Se-dependent hormone, was not altered by treatments (P>0.12) prior to AI but, during gestation, T3 decreased markedly (P<0.03) in all groups. However, at 30 days of gestation, values for selenite were lower (P<0.02) than for Sel-Plex® (Figure 4). Such a depressant effect with dietary supplementation of inorganic selenium has also been reported in rats (Behne et al., 1992; Eder et al., 1995; Hawkes and Keim, 2003). Eder et al. (1995) found serum T3 levels to be lower in both Se-deficient animals (0.038 ppm of total dietary Se) and in those given high levels of dietary Se (over 0.6 ppm Se) as compared to those receiving 0.1 and 0.3 ppm inorganic Se. In the present experiment, the background level of selenium in the basal diet was sufficient to prevent the depressing effect on T3, but this was not apparently the case for the selenitesupplemented sows. The selenium metabolism related to T3 synthesis in early gestation was apparently altered by inorganic selenium but not by organic selenium at the same dietary levels. Such an effect deserves more thorough investigation, in particular with regard to the activity of the selenoenzyme involved (Type I iodothyronine deiodinase), which converts thyroxin (T4) to deiodothyronine (T3), the active form of thyroid hormone.
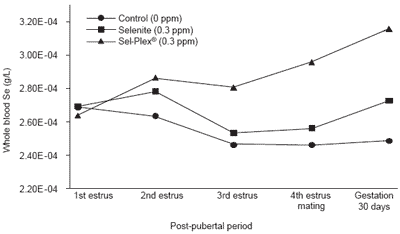
Figure 2. Blood selenium changes according to level and source of dietary selenium and post-pubertal period.
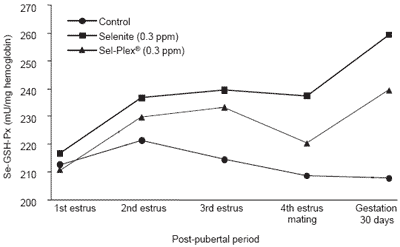
Figure 3. Blood Se-GSH-Px activity according to level and source of dietary selenium and post-pubertal period.
Short-term effects on selenium-related metabolites and hormone status during the peri-estrus period (cannulated animals)
There was no treatment effect (P>0.16) on the profile of luteinizing hormone (LH) during the third estrus. This peak of LH being a reliable indicator of the time of estrus, it was used to determine the time of estrus (day 0) for all the other measurements. Blood selenium effects were similar to those reported for long-term blood samplings. However, for Se-GSH-Px, the effects were similar to the long-term status before the day of estrus. Values were higher (P<0.03) for the selenite supplemented group than for the Sel-Plex® group from day -3 to day 0. However, the effect changed markedly thereafter; in fact, Se-GSH-Px values dropped in control sows and were lower (P<0.05) in controls than in Sesupplemented groups from day -2 to day +3 (Figure 5).
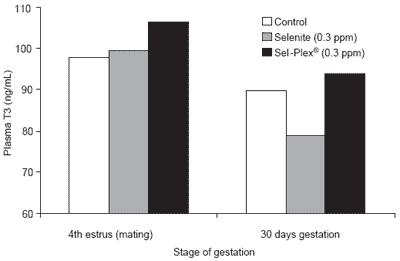
Figure 4. Plasma T3 changes according level and source of dietary selenium and stage of gestation.
It seems that there was an increased metabolic demand for Se-GSH-Px synthesis during and after estrus. The supply of selenium appeared insufficient for Se-GSHPx homeostasis in control sows, whereas the high level of accumulated selenium in Sel-Plex® sows allowed an increased synthesis of that enzyme at the time surrounding ovulation. Such response supports the hypothesis of an increased oxidative pressure and an increased transient and local demand for antioxidant at the time of ovulation.
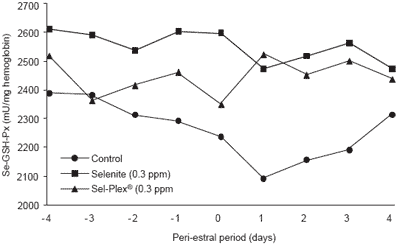
Figure 5. Blood Se-GSH-Px activity according to level and source of dietary selenium and peri-estral period (3rd estrus).
Plasma FSH was lower (P<0.04) for selenite-treated sows than for controls on day +2 and day +3. On day +4, FSH values (C: 0.88 ± 0.12, selenite: 0.94 ± 0.19 and Sel-Plex®: 1.27 ± 0.20 ng/mL) and T3 values (C: 87.5 ± 11.6, selenite: 75.3 ± 6.8 and Sel-Plex®: 96.8 ± 16.1 ng/100 mL) became higher (FSH, P<0.05) or tended to be higher (T3, P<0.07) for Sel-Plex® than selenite. Taking into account the possible role of T3 in the gene expression of FSH in other metabolic pools (Lerrant et al., 1988), those FSH and T3 responses after estrus merit further investigation. The profile of estradiol 17-ß (E2) was altered by the treatments (Figure 6). The E2 concentration preceding estrus, which included the pre-ovulatory peak, was lower in selenite sows than in Sel-Plex® sows (P<0.01). This response could be related to the GSH-Px response, since the intracellular GSH-Px would be up-regulated by estradiol (Lapointe et al., 2005; Bilodeau, personal communication). Therefore, it is tempting to suggest that the GSH-Px response around the third estrus in the Sel-Plex® group was due to E2 stimulation of GSH-Px synthesis where, in control sows, the E2 regulation of GSH-Px would have been repressed by a lack of metabolically available selenium. In selenite sows, as suggested previously, the inorganic selenium might have previously maximized the GSH-Px response, which stayed high and constant throughout the sampling period in spite of its lower E2 peak.
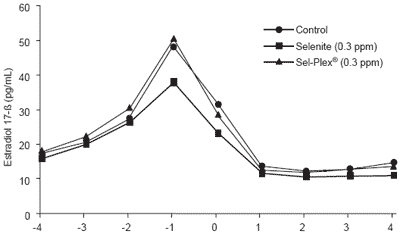
Figure 6. Plasma estradiol 17-ß according to level and source of dietary selenium and the peri-estral period (3rd estrus).
Reproductive performance on day 30 of gestation
On days 14 and 15 following the third estrus, sows received a hormonal challenge to synchronize (Lutalyse (10 mg Dinoprost)) and stimulate ovulation (P.G. 600 (400 UI PMSG et 200 UI HCG)). This was done to mimic the ovulation rate and eventually the reproductive performance of hyperprolific sows. The conception rate was affected by the hormonal challenge since, at slaughter, data and samples of embryos and corpora lutea (CL) were collected from 35 gravid sows (controls =12, selenite=13 and Sel-Plex®=10).
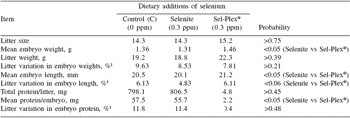
There was no treatment effect on mean litter size(P>0.75), but the average length, weight and protein content of embryos were respectively higher by 5.5%, 11.5% and 11.6% in Sel-Plex® than selenite sows (P<0.05) (Table 2). The transfer of selenium to embryos was also drastically affected by dietary selenium.
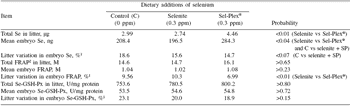
Although the selenium content of individual embryos was higher for the two groups of Se-treated sows than for controls (P<0.04) (Table 3), the difference between the two sources of dietary selenium was particularly marked. Indeed, in Sel-Plex® sows, the total litter and individual selenium content of embryos were 62.6% and 44.7% higher, respectively, than in selenite sows (P<0.01) (Table 3). Such a response to organic vs inorganic selenium has been reported previously in neonatal piglets by Mahan and Kim (1996); Kim and Mahan (2001) and by Mahan and Peters (2004) after longer periods of dietary selenium supplementation. However, in contrast to the present experiment, other studies have reported a response to inorganic selenium as compared to a control (<0.1 ppm background Se) (Hostetler and Kincaid, 2004; Mahan and Peters, 2004).
Although the discrepancy could be linked to the levels of dietary selenium in the control treatments (>0.2 ppm in the present case vs <0.1 ppm in Hostetler and Kincaid 2004) and Mahan and Peters (2004)), it is also possible that transfer of selenium to fetuses occurred later in gestation with the dietary inorganic selenium but not with the organic source. If this is the case, such a delay appeared rather critical since embryo development as evaluated by both morphological (length and weight) and analytical (protein) criteria was impaired in both control and selenite sows compared with those receiving Sel-Plex® selenium. In previous reports (Mahan and Kim, 1996; Mahan and Peters, 2004), there was no marked effect of the source of dietary selenium on postnatal growth of piglets. It appears that the hyperprolificacy induced in the present experiment has increased considerably the transient and local (ovary and/or uterus) tissue requirement for selenium as suggested by the results (in particular those of control sows) on GSH-Px profiles during the third estrus. The dietary selenium provided to Sel-Plex® sows appeared to be the only source able to cope with the oxidative pressure and to maximize the quality of ovulation and the subsequent development of embryos. In this way, the total antioxidative capacity (FRAP) at the embryo level was more uniform within Sel-Plex® than selenite litters (P<0.01) (Table 3). In general, there were, in fact, few treatment effects on within-litter variation. Besides FRAP in embryos, there was also a trend (P=0.06) in favor of selenite (4.8%) as compared to control and Sel-Plex® sows (6.1%) (Table 2) for less within-litter variation (more uniformity) in embryonic length; however it should be noted that such small values (=6.1%) represent, overall, very uniform litters.
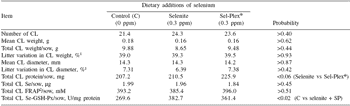
As far as corpora lutea are concerned, there was no treatment effect (P>0.18) on weight, diameter, total number, selenium or FRAP content (Table 4). However, GSH-Px in CL was higher for Se-supplemented than for control sows (P=0.02) (Table 4). The protein content of CL tended to be higher for Sel-Plex® than selenite (P=0.06) (Table 4). The latter effects on the CL need further study in order to determine whether the Sel- Plex® effects on embryo development originated from ovulation.
Table 2. Reproductive performance and embryonic development at 30 days of gestation according to dietary selenium source.
To enlarge the image, click here
1This value corresponds to the ‘within litter coefficient of variation’ ((within litter standard deviation for weight, length or protein ÷ within litter mean of embryo weight, length or protein) x 100))
Table 3. Selenium transfer to embryos and their antioxidant status at 30 days of gestation according to dietary selenium source.
To enlarge the image, click here
1This value corresponds to the “within litter coefficient of variation” ((within litter standard deviation for weight, length or protein ÷ within litter mean of embryo weight, length or protein) x 100))
2Ferric reducing antioxidant power
Table 4. Development, selenium transfer and antioxidant status in corpora lutea (CL) at 30 days of gestation according to dietary selenium source.
To enlarge the image, click here
1This value corresponds to the ‘within litter coefficient of variation’ ((within litter standard deviation for weight, length or protein ÷ within litter mean of embryo weight, length or protein) x 100))
2Ferric reducing antioxidant power
Conclusions
In summary, the selenium status response was affected by both the level and the type of dietary selenium supplement. Organic selenium (Sel-Plex®) substantially increased blood selenium status of sows as compared to selenite. Those effects were not, however, reflected in GSH-Px, which responded to type of selenium supplement. The results suggest that in ‘hyperprolifictype’ sows, dietary organic selenium would be necessary to optimize the T3 response of sows during gestation. Moreover, in such conditions, dietary organic selenium would be critical for an efficient selenium transfer to embryos and for their subsequent development in early gestation. Those effects of organic selenium on embryo development could possibly originate from treatment effects at the time of ovulation.
Acknowledgments
The authors would like to thank M. Guillette, V. Fauteux for technical assistance, the animal care team under supervision of D. Morrisette and D. Boyaud, Aliments Breton for professional assistance. The financial support for that project was provided by Alltech, Nicholasville, KY, USA and Aliments Breton, Qc, Canada.
References
Aten, R.F., K.M. Duarte and H.R. Behrman. 1992. Regulation of ovarian antioxidant vitamins, reduced glutathione, and lipid peroxidation by luteinizing hormone and prostaglandin F2 alpha. Biol. Reprod. 46:401-407.
Aten, R.F., T.R. Kolodecik and H.R Behrman. 1994. Ovarian vitamin E accumulation: evidence for a role of lipoproteins. Endocrinology 135:533-539.
Behne, D., A. Kyriakopoulos, H. Gessner, B. Walzog and H. Meinhold. 1992. Type I iodothyronine deiodinase activity after high selenium intake, and relations between selenium and iodine metabolism in rats. J. Nutr. 122:1542-1546.
Driancourt, M.A., F. Martinat-Botté and M. Terqui. 1998. Contrôle du taux d’ovulation chez la truie:l’apport des modèles hyperprolifiques. INRA Prod. Anim. 11:221-226.
Eder, K., A. Kralik and M. Kirchgessner. 1995. Effect on metabolism of thyroid hormones in deficient to subtoxic selenium supply levels. Z Ernahrungswiss. 34:277-283.
Flohé, L., E. Wingender and R. Brigelius-Flohé. 1997. Regulation of glutathione peroxidases. In: Oxidative Stress and Signal Transduction (H. Forman and E Cadenas, eds). Chapman and Hall, New York, NY, pp. 415-440.
Gondret, F., L. Lefaucheur, I. Louveau, H. Juin and B. Lebret. 2005. Influence du poids des porcelets à la naissance sur la composition des carcasses et des muscles au poids commercial d’abattage et la qualité sensorielle de la viande. Journées Rech. Porcine en France. 379:129-134.
Hawkes, W.C. and N.L. Keim. 2003. Dietary selenium intake modulates thyroid hormone and energy metabolism in men. J. Nutr. 133:3443-3448.
Hostetler, C.E. and R.L. Kincaid. 2004. Materna selenium deficiency increases hydrogen peroxide and total lipid peroxides in porcine fetal liver. Biol. Trace Elem. Res. 97:43-56.
Kim, Y.Y. and D.C. Mahan. 2001. Prolonged feeding of high levels of organic and inorganic selenium to gilts from 25 kg body weight through one parity. J. Anim. Sci. 79:956-966.
Lapointe, J., S. Kimmins, L.A. MacLaren and J.F. Bilodeau. 2005. Estrogen selectively up-regulates the phospholipid hydroperoxide glutathione peroxidase (PHGPx) in the oviducts. Endocrinology 146:(in press).
Le Cozler, Y., X. Pichodo, H. Roy, C. Guyomarc’h, H. Pellois, N. Quiniou, I. Louveau, B. Lebret, L. Lefaucheur and F. Gondret. 2004. Influence du poids individuel et de la taille de la portée à la naissance sur la survie du porcelet, ses performances de croissance et d’abattage et la qualité de la viande. Journées Rech. Porcine en France 36:443-450.
Legault, C. 1998. Génétique et prolificité chez la truie: la voie hyperprolifique et la voie sino-européenne. INRA Prod. Anim. 11:214-218.
Le Grusse, J. and B. Watier. 1993. Les vitamines, données biochimiques, nutritionnelles et cliniques. Centre d’étude et d’informations sur les vitamines, produits. Roche, Neuilly-sur-Seine, France.
Lerrant, Y., G. D’Angelo Bernard, M. Moumni and R. Counis. 1988. Ambivalent effect of thyroxine on the expression of gonadotropin genes in normal and orchidectomized rats. Pathol. Biol. (Paris) 36:973- 978.
Mahan, D.C. and Y.Y. Kim. 1996. Effect of inorganic or organic selenium at two dietary levels on reproductive performance and tissue selenium concentrations in first-parity gilts and their progeny. J. Anim. Sci. 74:2711-2718.
Mahan, D.C. and J.C. Peters. 2004. Long-term effects of dietary organic and inorganic selenium sources and levels on reproducing sows and their progeny. J. Anim. Sci. 82:1343-1358.
Matte, J.J., W.H. Close and C. Pomar. 1991. The effect of interrupted suckling and split-weaning on reproductive performance of sows: a review. Livestock Prod. Sci. 30:195-212.
McDowell, L.R. 2000. Vitamins in Animal Nutrition. Academic Press Inc., San Diego, CA, USA. Murray, A.A., M.D. Molinek, S.J. Baker, F.N. Kojima, M.F. Smith, S.G. Hillier and N. Spears. 2001. Role of ascorbic acid in promoting follicle integrity and survival in intact mouse ovarian follicles in vitro. Reproduction 121:89-96.
Petroff, B.K., K. Dabrowski, R.E. Ciereszko and J.S. Ottobre. 1997. Total ascorbate and dehydroascorbate concentrations in porcine ovarian stroma, follicles and corpora lutea throughout the estrous cycle and pregnancy. Theriogenology 47:1265-1273.
Petroff, B.K., R.E. Ciereszko, K. Dabrowski, A.C. Ottobre, W.F. Pope and J.S. Ottobre. 1998. Depletion of vitamin C from pig corpora lutea by prostaglandin F2 alpha-induced secretion of the vitamin. J. Reprod. Fertil. 112:243-247.
Rose, U.M., R.G.J.M. Hanssen and H.J. Kloosterboer. 1999. Development and characterization of an in vitro ovulation model using mouse ovarian follicles. Biol. Reprod. 61:503-511.
Segerson, Jr., E.C., F.A. Murray, A.L. Moxon, D.R. Redman and H.R. Conrad. 1977. Selenium/vitamin E: role in fertilization of bovine ova. J. Dairy Sci. 60:1001-1005.
Segerson, E.C. and S.N. Ganapathy. 1980. Fertilization of ova in selenium/vitamin E-treated ewes maintained on two planes of nutrition. J. Anim. Sci. 51:386-94.
Tarin, J., J. Ten, F.J. Vendrell, M.N. de Oliveira and A. Cano. 1998. Effects of maternal ageing and dietary antioxidant supplementation on ovulation, fertilisation and embryo development in vitro in the mouse. Reprod. Nutr. Dev. 38:499-508.
Tribout, T., J.C. Caritez, J. Gogue, J. Gruand, Y. Billon, M. Bouffaud, H. Lagant, J. LeDividich, F. Thomas, H. Quesnel, R. Guéblez and J. P. Bidanel. 2003. Estimation, par utilisation de semence congelée, du progrès génétique réalisé en France entre 1977 et 1998 dans la race porcine Large White: résultats pour quelques caractères de reproduction femelle. Journées Rech. Porcines en France 35:285-292.
Authors: MARIE-ÈVE FORTIER1,2 and J. JACQUES MATTE2
1 Université Laval, Ste-Foy, Quebec, Canada, and
2 Agriculture and Agri-Food Canada, Lennoxville, Quebec, Canada
With the invaluable scientific collaboration of Hélène Quesnel, Institut de la Recherche Agronomique, St-Gilles, France, Jean-François Bilodeau1, Alain Giguère2 and Jean-Paul Laforest1






