Detection of antibodies against pathogens in feral and domestic pigs (Sus scrofa) at the Sierra La Laguna Biosphere Reserve, Mexico
Several diseases that were believed to be controlled or eradicated have reappeared and have had catastrophic effects on humans and on domestic and wild animals. Approximately 60 % of recently registered disease outbreaks are caused by zoonotic agents, and 72 % of these originated in a wild species. Swine (Sus scrofa) is a species that favors the propagation of pathogens, and they can be a reservoir of many diseases. Thus, the objective of this study was to detect the presence of viral and bacterial diseases that could impair the health of wild animals and humans in both feral and domesticated pigs at the Sierra La Laguna Biosphere Reserve. Diagnosis was performed with serological tests on 70 animals to detect antibodies against swine influenza virus (SIV), porcine respiratory reproductive syndrome virus (PRRSV), Aujeszky’s disease virus (ADV), leptospirosis (Lp), salmonellosis (Sal), and brucellosis (Br).
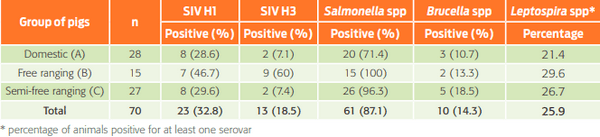
No antibodies were detected against PRRS or AD, whereas the seropositivity was 30.7 % for SI, 25.9 % for Lp, 87.1 % for Sal, and 14.3 % for Br. This evidence supports the presence of these pathogens in Sierra Laguna, and implies that swine could be an important factor for transmission to other wild species, as well as to people who have had contact with or consumed these animals. Thus, developing management and epidemiological surveillance plans for the animals inhabiting the region is of paramount importance.
Keywords: Feral swine; zoonosis; antibodies; Mexico.
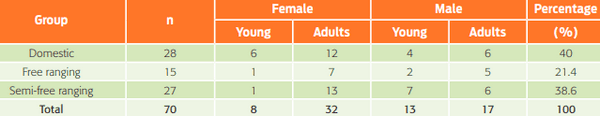
- Domestic pigs (Group A); pigs kept inside a barn or another facility and fed by their owners.
- Free ranging pigs (Group B); pigs roaming mostly free that obtained food by themselves. They reproduced naturally, without any type of handling.
- Semi-free ranging pigs (Group C); feral pigs that had been used for consumption and were fed by farmers. Seventy animals were used for the study. The sample size was based on an expected prevalence of 5 % with a statistical error of 5 %19. The sex and age of the pigs are shown in Table 1.
- The hemagglutination inhibition test (HI) was used for the diagnosis of swine influenza following the protocol in Beltrán (2009)22, which is considered the standard test for this disease by the World Organization for Animal Health23 (OIE for its acronym in French, http://www.oie.int/es/ retrieved 24/06/2012). The viruses A/swine/NewJersey/11/76 (H1N1) and A/swine/Minnesota/9088-2/98 (H3N2) were used as the antigens, and sera that showed sedimentation in a dilution greater than or equal to 1:80 were considered positive.
- ELISA tests were used for the diagnosis of Aujeszky’s disease (Hipra CIVTEST SUIS ADVgE No. CAE.12, Spain); PRRS: an indirect ELISA test was used (Hipra CIVTEST SUIS PRRS A/S No. 40ND, Spain); and for Salmonellosis, Idexx was used (Idexx - SWINE SALMONELLA HERD CHEK No. 44100 T 161, USA). Idexx detects antibodies for the most common Salmonella serotypes and indicates the exposure of the herd to these bacteria. The manufacturer’s protocols were followed to perform and interpret each assay.
- Microscopic microagglutination (MAT) was used to diagnose leptospirosis. It is the reference test given by OIE, and antigens representative of the area inhabited by the animals were used23,24. Nine Leptospira interrogans serovarieties from the collection of CENID-Microbiología were used. This test was scored as positive starting at a 1:100 dilution based on the minimum significant titer of the OIE.
- Card Test (3 %), an indirect diagnostic test also known as Rose Bengal, was used for the serological diagnosis of brucellosis. The antigen Brucella abortus strain 1119-3 at 8 % was used for the specific diagnosis of brucellosis in swine. It was stained with Rose Bengal from the Productora Nacional de Biológicos Veterinarios (PRONABIVE) which detects B. abortus, B. suis, and B. melitensis. This test was performed following the manufacturer’s instructions.
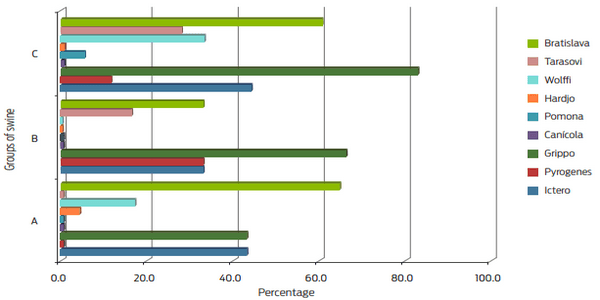
1. Petersen EA. Emerging infectious disease. Arch Intern Med. 1996;156:124. doi: 10.1001/archinte.1996.00440020010001.
2. Daszak P. Emerging infectious diseases of wildlife-- Threats to biodiversity and human health. Science. 2000;287:443-9. doi: 10.1126/science.287.5452.443.
3. FAO-OIE-WHO. Influenza and other emerging zoonotic diseases at the human-animal interface. Food and Agriculture Organisation of the United Nations - World Organisation for Animal Health - World Health Organisation ed. Verona, Italy2010 2010.
4. Meng XJ, Lindsay DS, Sriranganathan N. Wild boars as sources for infectious diseases in livestock and humans. Phil Trans R Soc B: Biol Sci. 2009;364:2697- 707. doi: 10.1098/rstb.2009.0086.
5. Seward N, VerCauteren K, Witmer G, Engeman R. Feral swine impacts on agriculture and the environment. Sheep Goat Res J. 2004;19:34-40.
6. Gibbs EPJ. The public health risks associated with wild and feral swine. Rev Sci Tech - Off Int Epizoot. 1997;16:594-8. doi: 10.20506/rst.16.2.1052.
7. Vitousek P, D´Antonio C, Loope L, Rejmánek M, Westbrooks R. Introduced species: a significant component of human-caused global chance. N Z J Ecol. 1997;21:1-16.
8. Hutton T, DeLiberto T, Owen S, Morrison B. Disease risks associated with increasing feral swine numbers and distribution in the United States. USA: Midwest Association of Fish and Wildlife Agencies; 2006 7 Nov.
9. Ortega-Rubio A, Lagunas-Vázques M, Beltrán-Morales LF. Evaluación biológica y ecológica de la Reserva de la Biósfera Sierra la Laguna, Baja California Sur: Avances y retos. Ortega-Rubio A, Lagunas-Vázques M, Beltrán-Morales LF, editors. La Paz, BCS: Centro de Investigaciones Biológicas del Noroeste, SC; 2012. 422 p.
10. CONABIO. Programa de manejo de la Reserva de la Biósfera Sierra La Laguna. Comisión Nacional de Áreas Naturales Protegidas DGdMplC, editor. México: Comisión Nacional de Áreas Naturales Protegidas; 2003. 208 p.
11. Arnaud G, Álvarez S, Cortés P. Mamíferos de la Reserva de la Biósfera Sierra la Laguna. In: Ortega-Rubio A, Lagunas-Vázques M, Beltrán-Morales LF, editors. Evaluación de la Reserva de la Biósfera Sierra la Laguna, Baja California Sur: Avances y Retos. La Paz: Centro de Investigaciones Biológicas de Baja California Sur A.C.; 2012. p. 412.
12. Breceda A, Arnaud G, Álvarez S, Galina P, Montes J. Evaluación de la población de cerdos asilvestrados (Sus scrofa) y su impacto en la Reserva de la Biosfera Sierra La Laguna , Baja California Sur , México. Trop Conserv Sci. 2009;2:173-88.
13. Montes-Sánchez J, León de la Luz JL, Buntinx-Dios S, Aguilar-Marcelino L, Blázquez-Moreno MC. Dieta, crecimiento y reproducción del cerdo asilvestrado Sus scrofa en la Reserva de la Biósfera Sierra la Laguna. In: Ortega-Rubio A, Lagunas- Vázques M, Beltrán-Morales LF, editors. Evaluación de la Reserva de la Biósfera Sierra la Laguna, Baja California Sur: Avances y Retos. La Paz: Centro de Investigaciones Biológicas del Noroeste S.C.; 2012. p. 183-204.
14. Glass CM, McLean RG, Katz JB, Maehr DS, Cropp CB, Kirk LJ, et al. Isolation of pseudorabies (Aujeszky’s disease) virus from a florida panther. J Wildlife Dis. 1994;30:180-4. doi: 10.7589/0090-3558-30.2.180.
15. Hall JS, Minnis RB, Campbell TA, Barras S, DeYoung RW, Pabilonia K, et al. Influenza exposure in United States feral swine populations. J Wildlife Dis. 2008;44:362-8. doi: 10.7589/0090-3558-44.2.362.
16. Raymond JT, Gillespie RG, Woodruff M, Janovitz EB. Pseudorabies in captive Coyotes. J Wildlife Dis. 1997;33:916-8. doi: 10.7589/0090-3558-33.4.916.
17. Kirkpatrick CM, Kanitz CL, McCrocklin SM. Possible role of wild mammals in transmission of pseudorabies to swine. J Wildlife Dis. 1980;16:601-14.
18. Arriaga L, Ortega A. La Sierra de la Laguna de Baja California Sur. Arriaga L, Ortega A, editors. La Paz: Centro de Investigaciones Biológicas de Baja California Sur A.C.; 1989. 237 p.
19. Cortés F. Tamaño de muestra y análisis de asociación. Rev Mex Sociol. 1982;44(4):1381-411. doi: 10.2307/3540134.
20. Straw BE, Meuten DJ, Thacker BJ. Physical examination. In: Straw B, D’Allaire S, Mengeling W, Taylor D, editors. Diseases of swine. 8 ed. Iowa, USA: Iowa State University Press/ Ames; 1999. p. 15-7.
21. Segalés J, Martínez J, Catellà J, Darwich L, Domingo M, Mateu E, et al. Manual de diagnóstico laboratorial porcino. MSD SA, editor. Navarra, España: Servet; 2013. 120 p.
22. Beltrán Figueroa R. Identificación del virus de influenza porcina subtipos H1N1 y H3N2 mediante RT-PCR [Tesis de licenciatura]. Ciudad de México: Universidad Nacional Autónoma de México; 2009.
23. OIE. Manual de las pruebas de diagnóstico y de las vacunas para los animales terrestres. 7 ed. Paris, France: Office International des Epizooties; 2012. 1404 p.
24. Olsen S. Porcine brucellosis. OIE Manual of diagnostic tests and vaccines for terrestrial animals. 2. 6 ed. Paris, France: Office International des Epizooties; 2008. p. 1343.
25. Gaston W, Armstrong JB, Arjo W, Stribling HL. Home range and habitat use of feral hogs (Sus scrofa) on Lowndes Country WMA, Alabama. National conference on feral hogs April 13-15; MO, USA2008. p. 6.
26. Ruiz-Fons F, Vidal D, Vicente J, Acevedo P, Fernández-de-Mera IG, Montoro V, et al. Epidemiological risk factors of Aujeszky’s disease in wild boars (Sus scrofa) and domestic pigs in Spain. Eur J Wildl Res. 2008;54:549-55. doi: 10.1007/ s10344-008-0179-6.
27. Vicente J, León-Vizcaíno L, Gortázar C, Cubero MJ, González M, MartínAtance P. Antibodies to selected viral and bacterial pathogens in european wild boars from Southcentral Spain. J Wildlife Dis. 2002;38:649-52. doi: 10.7589/0090-3558-38.3.649.
28. Ruiz-Fons F. Riesgos sanitarios asociados a la producción cinegética del jabalí: la enfermedad de Aujeszky [Tesis de doctorado]. Ciudad Real, España: CSICUCLM - Instituto de Investigación en Recursos Cinegéticos (IREC) Universidad de Castilla-La Mancha; 2006.
29. Montagnaro S, Sasso S, De Martino L, Longo M, Iovane V, Ghiurmino G, et al. Prevalence of antibodies to selected viral and bacterial pathogens in wild boar (Sus scrofa) in Campania region, Italy. J Wildlife Dis. 2010;46:316-9. doi: 10.7589/0090-3558-46.1.316.
30. Thakur S, Sandfoss M, Kennedy-Stoskopf S, DePerno CS. Detection of Clostridium difficile and Salmonella in feral swine population in North Carolina. J Wildlife Dis. 2011;47:774-6. doi: 10.7589/0090-3558-47.3.774.
31. Vengust G, Valencak Z, Bidovec A. A serological survey of selected pathogens in wild boar in Slovenia. J Vet Med B. 2006;53:24-7. doi: 10.1111/j.1439-0450.2006.00899.x.
32. Wyckoff aC, Henke SE, Campbell Ta, Hewitt DG, VerCauteren KC. Feral swine contact with domestic swine: a serologic survey and assessment of potential for disease transmission. J Wildlife Dis. 2009;45:422-9. doi: 10.7589/0090-3558-45.2.422.
33. College of Veterinary Medicine Iowa State University. Swine diseases manual. 4 ed. Neuman EJ, Ramirez A, Schwartz KJ, editors. Iowa: American Association of Swine Veterinarians; 2013. 173 p.
34. Barrios-Garcia MN, Ballari SA. Impact of wild boar (Sus scrofa) in its introduced and native range: a review. Biol Invasions. 2012;14:2283-300. doi: 10.1007/ s10530-012-0229-6.
35. Kaden V, Lange E, Hänel A, Hlinak A, Mewes L, Hergarten G, et al. Retrospective serological survey on selected viral pathogens in wild boar populations in Germany. Eur J Wildl Res. 2009;55:153-9. doi: 10.1007/s10344-008-0229-0.
36. Boadella M, Ruiz-Fons JF, Vicente J, Martín M, Segalés J, Gortazar C. Seroprevalence evolution of selected pathogens in iberian wild boar. Transbound Emerg Dis. 2012;59:395-404. doi: 10.1111/j.1865-1682.2011.01285.x.






