Comparative toxic effects of dietary organic and inorganic selenium fed to swine and their implications for human nutritional safety
Published: November 2, 2006
By: DON MAHAN1 AND YOO YONG KIM2 - Alltech Inc.
Historically, the identification of selenium (Se) as a toxic element was indirectly referred to in at least two major writings. In both cases the detrimental effects of the element were unknown at the time of the
recordings. The first written account, quoted by Moxon (1937), was from Marco Polo who traveled the Orient during the 13th century where he reported:
“.....It is a fact that when they take that road, they cannot venture amongst the mountains with any beasts of burden excepting those accustomed to the country, on account of a poisonous plant growing there, which if eaten by them, has the effect of causing the hoofs of the animals to drop off; but those of the country being aware of its dangerous quality, take care to avoid it......”
The second writing occurred in the United States, where homesteaders of the Great Plains area had encountered lameness problems with their livestock. These settlers were the original group who coined the term ‘alkali disease’ as they had suspected that the alkaline (high salt) waters of the semi-arid area, or the alkali seeps and alkali spots in the soil, caused a lameness disease in their livestock. Obviously, later work did not confirm the condition to be associated with these alkali deposits, but the term ‘alkali disease’ continued in use (Moxon, 1937). It was in the report of an army surgeon (Madison) at Camp Randall (Territory of Nebraska) who in 1856 issued a congressional report that revealed the situation (Moxon, 1937):
“A very fatal disease manifested itself among the dragoon horses which is supposed not to have been described in works on veterinary surgery. Four companies of the second dragoon arrived at this post about the 10th of August, 1856........ The four companies encamped on the east or lower side of the dry ravine separating the dragoon and infantry camps. About the 20th of August the disease commenced simultaneously in all four companies and many horses died, not, however, until the lapse of weeks and months. The following symptoms were observed...... After forage was provided for the horses no new
cases occurred.”
The report of Madison outlined the clinical signs that we now recognize as the classical signs of selenium intoxication, but the principal ones were a severe tenderness and abnormal growth of the hoof resulting in an inability to adequately graze. This resulted in an emaciated animal, the same conditions as denoted by Marco Polo.
It is also of interest to note that other historians have reported that in the area of the Great Plains, and particularly in the Dakotas where Indians and U.S. Army skirmishes frequently occurred, that Indian ponies could move about more freely and they often outran the Army mounts (Anonymous, 1999). The Indian ponies were not fenced in for grazing as were the army horses, and it is thus possible that during periods of drought (common in this semi-arid area) the Army horses were forced to consume forages perhaps containing the ‘poisonous’ element. Consequently, their feet or hooves were sore and movement was somewhat painful. The outcome of many battles including the most famous one by General George Custer (Little Big Horn) in 1876 may have been indirectly influenced by the element selenium. Much of the early pioneering effort in identifying the toxic effects of selenium was investigated by South Dakota researchers, Drs. Franke, Moxon and Olson, in the early decades of the 1900s. Moxon (1937) and Miller and Schoening (1938) subsequently reported that selenosis in swine involved hair loss, cracking of hooves, and an interruption in the coronary band development of the hoof.
Acute and chronic toxicosis (selenosis)
The toxic effects of selenium can generally be classified into two types depending upon the amount of selenium ingested and the duration of its administration. Acute selenosis occurs after an extremely high dietary intake ($20 mg/kg) or injection ($1.65 mg/kg BW) of selenium (Miller and Williams, 1940; Diehl et al.; 1975; Mahan and Moxon, 1984) with death often occurring within hours. Acute selenosis thus results in respiratory distress, ataxia, diarrhea, and frequently death. Chronic selenosis on the other hand occurs after consuming diets that contain 5 to 20 ppm Se over a longer time period (Goehring et al., 1984a,b; Mahan and Moxon, 1984). A reduced feed intake and growth rate appear to be the more immediate indicators of chronic selenosis. Additional signs include hair loss, sloughing of hooves, liver cirrhosis, and anemia (Ekermans and Schneider, 1982).
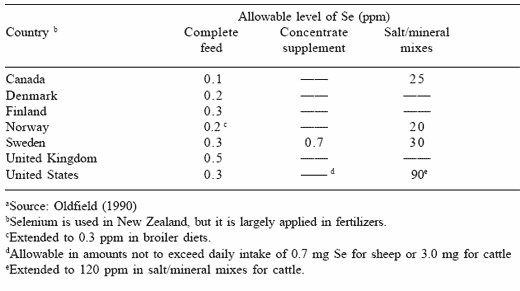
Although toxicity problems were the initial concerns of the element, selenium has now been recognized as a dietary essential for both animals and humans. However, because of the problems emanating from its original toxicity effects, and an erroneous report indicating that selenium was carcinogenic, the supplemental level of the element is now regulated by governments throughout the world. The current approval level for dietary selenium in several countries is reported in Table 1. Generally the approval level ranges from 0.1 to 0.5 ppm of supplemental selenium in the complete diet mixture, levels that are 30 to 50 times below the toxicity responses reported by several researchers. In much of the world both the organic or inorganic selenium forms can be added to livestock diets, whereas in some countries only the organic or the inorganic forms are currently approved as the supplemental source of selenium.
Table 1. Approved levels of selenium supplementation in feed a
Selenosis: organic versus inorganic selenium sources
SHORT-TERM CHRONIC TOXICOSIS
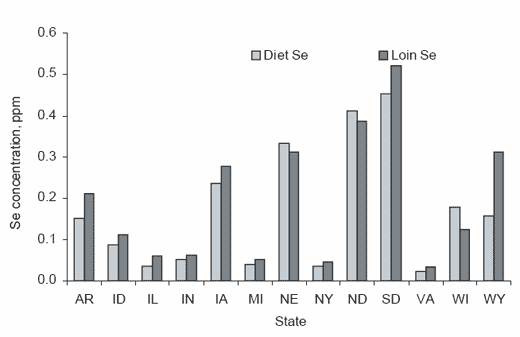
Although early reports of selenium toxicosis largely reflected the consumption of selenium originating from plant tissue, most of the controlled toxicity studies have used inorganic sources to define the harmful levels in animals and humans. There is, however, an important distinction that must be made regarding the effects of these two forms as each will produce differing responses. It is important to recognize that the organic form of the element in grains and animal tissue is largely found as selenomethionine and is incorporated into the protein chain (Olson et al., 1970). The plant normally manufactures methionine (which contains sulfur). However, when selenium is absorbed from the soil the plant also synthesizes selenomethionine, which is identical in chemical structure to methionine except that selenium replaces the sulfur component of the molecule. The plant thus manufactures both forms of methionine and both are placed into the protein component of the grain. The digestibility of proteins that contain selenomethionine (or methionine) from the various feed sources (e.g., grains, yeast, animal products, etc.) depends upon the proteolytic digestion processes of the animal and subsequent amino acid absorption mechanisms. The dietary level of both amino acids (methionine and selenomethionine) in the grain or protein source reflects their proportions in the feed protein source, and thus varies by the amount of selenium in the soils (Figure 1). Because methionine is an essential amino acid, the body cannot manufacture this amino acid or its substitute selenomethionine. The body mechanisms also cannot differentiate between methionine and selenomethionine and the one in greater supply in the body pool will be incorporated into the protein chain of the muscle and other body tissues in proportion to its supply in the body pool. The inorganic form of the element most frequently used in livestock and human diet is sodium selenite, a water-soluble form that is passively absorbed through the villi of the small intestine (Wolffram, 1999). Both forms can be effectively used for the formation of essential selenoproteins (e.g., glutathionine peroxidase, etc.) in the body, but they are not equally retained by body tissues.
Figure 1. Relationship of dietary and loin selenium concentration (Ku et al., 1972).
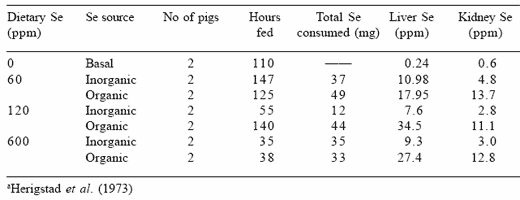
Herigstad et al. (1973) demonstrated that young 4-week-old pigs intravenously administered sodium selenite or selenomethione died sooner (2.5 hr vs. 14 hr) when sodium selenite was the selenium source. Subsequent research showed that when the organic form of the element was provided at dietary levels from 60 to 600 ppm Se, the acute response to high levels of selenium occurred with both forms, but was delayed and not as severe when the organic form was fed. Organic selenium was subsequently found to be retained at higher concentrations in the liver and kidney tissues within 150 hr of administration than was inorganic selenium (Table 2). The higher tissue retention of selenium in the organically fed group could result in a lower amount being circulated to the target tissues, which will thus delay the onset of selenosis.
Table 2. Short-term effects of organic (selenomethionine) or inorganic (sodium selenite) selenium fed to young pigs on tissue selenium concentrations a
Long-term studies with growing-finishing pigs evaluating the effects of organic selenium (Sel-Plex, Alltech Inc.) vs. inorganic selenium (sodium selenite) demonstrated that pig growth rates were similar when either form was added to pig diets at #5 ppm Se. When dietary levels exceeded 5 ppm Se, the detrimental effects of the inorganic selenium on both growth and feed intakes were more evident (Figure 2). At 20 ppm Se, pig gains were approximately seven times greater when the organic (0.55 vs. 0.08 kg/day) than when the inorganic form was provided.
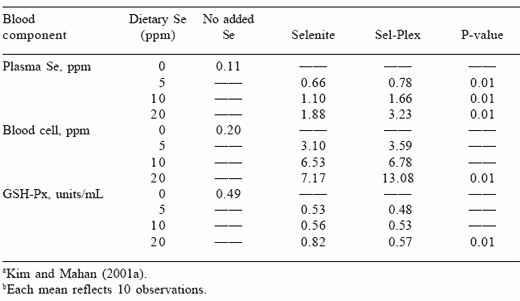
The results presented in Tables 3 and 4 suggest that when organic selenium was fed to pigs for a 12-week period, more of the organic element, probably as selenomethione or selenocysteine, was incorporated into body tissues. Higher red blood cell and plasma selenium concentrations also occurred when the organic form was fed with differences being greater between the two forms as the level of dietary selenium increased (Table 3). It is of interest to note that the glutathione peroxidase (GSH-Px) activity also increased when pigs were fed high dietary levels of either selenium form. The over production of GSH-Px by the body when excess selenium was provided suggests that it may be attempting to use the enzyme as an antioxidant to reduce the toxic effects of the element.
Figure 2. Effects of high dietary selenium fed to pigs on daily gain responses (Kim and Mahan, 2001a).
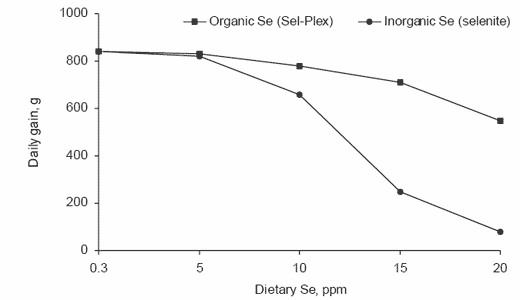
Table 3. Long-term effects of dietary organic or inorganic selenium on swine blood measurements after feeding for a 12-week period. a b
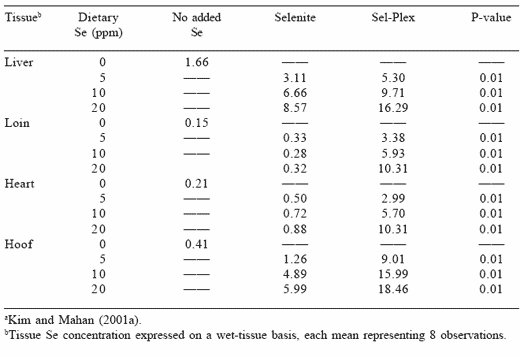
When the tissues of pigs fed the high levels of selenium were evaluated after the 12-week feeding period, those pigs fed organic selenium retained more selenium in soft tissues (liver, loin, heart) than the group fed inorganic selenium (Table 4). The loin tissue selenium concentration increased but plateaued when dietary inorganic selenium was fed but continued to increase as the dietary level of organic selenium increased. This indicated that not only was more of the organic selenium incorporated into the tissues as selenium increased, but that it was also effectively removed from the circulatory system. Consequently, the organic selenium source, although toxic at 5 ppm Se, produced less toxicity signs at the lower levels that when the inorganic form was fed. This is attributed to less of the organic selenium
being available for circulation to the various tissues to ultimately effect the onset of selenosis.
Table 4. Long-term effects of dietary organic or inorganic selenium on swine tissue selenium concentrations (ppm) a
LONG-TERM SOW STUDIES
Although excess organic selenium may not be as potentially harmful for grower-finisher pigs on various performance traits, its accumulation in the body may become more harmful in the adult body because of tissue turnover in the mature animal. Body tissue is routinely turned over or regenerated when production demands are high and tissue reserves (i.e., amino acids) are mobilized. With increased turnover at least part of the selenium incorporated into these tissues may be released into the circulatory system. This could result in detrimental effects when the animal is in the reproducing herd.
A study was conducted to evaluate the effects of feeding high levels of dietary selenium from 0.3 to 10 ppm Se from both selenium sources from 20 kg body weight through one parity. The performances of pigs prior to the reproductive phase are not reported here but are consistent with previous results (Kim and Mahan, 2001a).
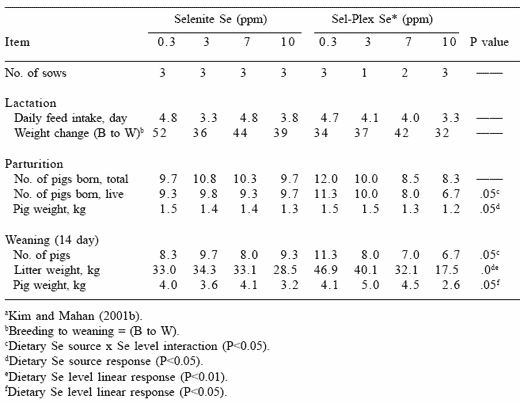
The two dietary selenium sources did not affect gestation or parturition performance of sows until the high levels of both sources were provided. When sows were fed organic selenium they seemed to suffer more from high dietary selenium than did sows consuming the inorganic selenium. Sows fed organic selenium particularly at dietary levels >7 ppm Se had lower numbers of pigs born and lower pig weights at both parturition and at weaning (Table 5). Consequently, organic selenium seemed to be more toxic to reproducing animals than the inorganic Se source. It is probable that tissue turnover from the organic selenium source may have contributed to the body’s circulating selenium pool, which in addition to the high level dietary level being fed caused a greater detrimental response in reproductive tissues.
Table 5. Long-term effects of dietary organic or inorganic selenium (fed from 20 kg body weight through one parity) on various sow reproductive measurements. a
Sow serum and milk selenium concentrations reported in Table 6 demonstrated that sows consuming the organic source had higher selenium concentrations in serum and both colostrum and later milks. These results clearly demonstrated that the organic selenium was effectively absorbed, transferred across mammary tissue, and incorporated into the milks of lactating sows. The milks of sows fed inorganic selenium were markedly lower in selenium content than sows fed the organic source. It is of interest to note that nursing pigs had hair losses and hoof separation when sows had been fed inorganic selenium at 7 ppm Se, but this did not occur until the organic selenium was fed to the sows at the 10 ppm Se level. This suggests that the form of selenium in the sow milks may have differed for the two sources and that the selenium appeared to be as, or perhaps more, toxic to nursing pigs when high levels of inorganic selenium were fed to lactating sows. The high feed intakes of lactating sows resulted in high quantities of
selenium being consumed, and when the body was unable to rid itself of the excess through the kidney some of the excess was ‘dumped’ into the milk. This excess ultimately increased the selenium supply to the nursing pig and the onset of selenosis (i.e., hair loss, hoof malformations).
Table 6. Long-term effects of dietary organic or inorganic selenium (fed from 20 kg body weight through one parity) on various sow blood and tissue selenium concentrations a
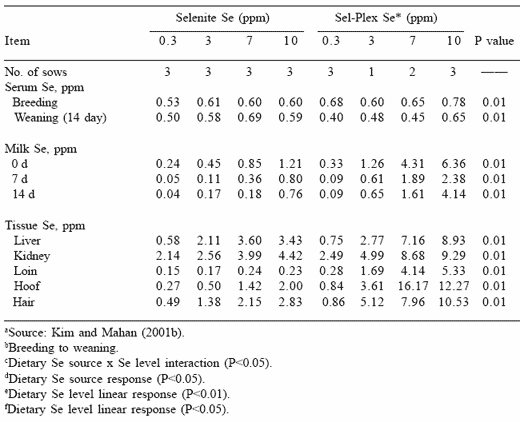
Tissue selenium concentrations increased when dietary selenium levels increased for both sources, but when sows were fed organic selenium the tissues had substantially higher selenium concentrations at each comparable dietary level (Table 6).
It is of interest to note that the hoof and hair selenium concentrations of sows fed organic selenium were substantially higher than in the soft tissues, particularly at the higher dietary selenium levels. Because the loss of hair and hoof material is characteristic of the selenosis condition, it is possible that these hard tissues could no longer handle the high selenium being retained and the tissues were therefore damaged and sloughed from the body. Serum selenium and GSH-Px activities were also higher in the neonatal pigs as the dietary selenium level fed to the sow increased (Table 7). These responses are consistent with previous reports demonstrating that both selenium forms effectively traverse the placenta and are deposited into fetal tissue. Although piglet serum GSH-Px activity was similar for both selenium groups at comparable sow dietary selenium levels, they tended to increase as the dietary level fed to the sow increased. At weaning, pig serum selenium concentrations were higher than at birth as the dietary selenium level increased, and were higher when sows were fed the organic form. Serum GSH-Px activities of weanling pigs were higher when the sow’s dietary selenium level increased, again suggesting an overproduction of the enzyme occurred when excess selenium was fed.
Table 7. Long-term effects of dietary organic or inorganic selenium (fed from 20 kg body weight through one parity) on various piglet tissue selenium measurements. a
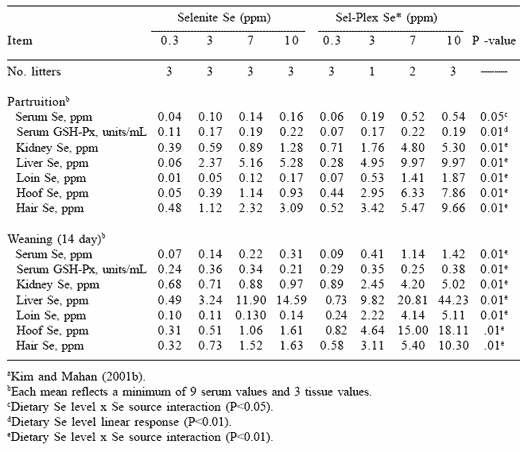
Tissue selenium concentrations were higher in the neonate when sows received the organic source, suggesting that this selenium source was more effectively retained in the tissues of the developing fetus. These responses are consistent with the responses in the grower-finisher pig and sow. Pig tissue selenium concentrations were higher at weaning than at birth, and were highest when the organic selenium source had been fed to the sows. These results confirm that the higher milk selenium concentrations from the organically-fed sows were effectively retained in the nursing pig and resulted in higher piglet selenium status at weaning.
It is of interest to note that the hair and hoof selenium concentrations of pigs at both parturition and weaning were higher than the other body tissues, and that these concentrations were particularly high when the sows had received the organic selenium. Sloughing of hoof and loss of hair occurred in pigs nursing sows fed the higher dietary selenium levels of either source.
What does all this mean to the pig?
Pigs as well as other animals rid themselves of excess selenium by clearing circulating selenium from the blood and excreting it in the urine by filtering it through the kidney. Because of the large supply, the kidney cannot eliminate all of the excess. Part of the excess remains in the circulating blood and is placed back into the endogenous secretions of the intestine through the bile where it is ultimately voided in the feces. When extremely high dietary selenium levels are fed some of the circulating selenium is also methylated as dimethyl selenide ([CH3]2Se) in the lung where it is expired. Often the breath smells of a garlic odor.
Clearly the results of these experiments demonstrate that both inorganic and organic selenium can be toxic for the pig at approximately 5 ppm Se. During the grower-finisher period, the effects are less noticeable when dietary selenium levels exceed 5 ppm Se. However, much of the organic selenium is effectively retained or entrapped in body protein tissues where it is not immediately available to precipitate tissue damage. Thus the selenosis condition is delayed. However, when extremely excessive levels are consumed (> 40 ppm) and the animal cannot get rid of the excess, or the animal’s tissue is saturated with selenium, the excess selenium can now cause rapid deterioration of tissues and cause emaciation and ultimately death. Inorganic selenium is not retained in the tissue and a higher proportion of it comes in direct contact with sensitive tissues resulting in more severe selenosis responses at lower dietary selenium levels.
Hoof and hair tissue show extensive damage and are subsequently lost from the animal’s body as the selenosis process advances. The data we collected suggest that selenium content of these tissues continues to increase as the dietary level increases. Hoof damage and loss of hair occur at the higher dietary levels from both selenium forms. In the case of inorganic selenium, this form does not accumulate at high concentration in many body tissues and therefore is either excreted or produces the selenosis condition at a lower dietary selenium level. Consequently, because much of the organic selenium is retained in the tissues, it takes longer for pigs to show the selenosis condition when provided at marginal toxic levels (e.g., 5 ppm Se). However, when dietary levels of organic selenium are fed in great excess or when the tissues become completely saturated or their storage capability is exceeded, the animal will be more severely affected than if inorganic selenium had been fed.
During gestation the restricted feed intake of sows fed high dietary selenium results in a reduction in the amount of selenium consumed. Consequently, the effects of selenosis would be expected to be less, particularly if the animal could rid itself of any excess consumed. But when tissues are already saturated with selenium, as from the organic source, and turnover of tissue selenium reservoirs occurs, then some of the tissue selenium becomes available and can be potentially detrimental to the animal. This is probably what happened when organic selenium was fed at the high level to pregnant sows. In that case sows fed the high dietary levels of organic selenium had lower litter sizes and lower reproductive responses. This was attributable to more of the organic selenium being released from tissue turnover to the circulation system. However, the amount of tissue selenium released when inorganic selenium was fed was minimal because of its lower tissue concentration.
During lactation the situation is somewhat different. The sow in this case is energy deficient and she consumes more feed to offset her energy deficiency. Being unable to rid the body of excess selenium from either source when the high dietary selenium levels are fed, the sow will now suffer from a higher selenium intake. Even though she attempts to rid the excess selenium through the urine some of it passes through the mammary gland and into the milk. Ultimately sow production, feed intake, and milk production will be affected. The nursing pig thus consumes and accumulates selenium because of the higher concentration in the milk. When the nursing pig consumes the high amount of milk selenium it will result in the selenosis response. Pigs of both sow groups had hair loss and hoof separation when they consumed the milk of sows fed the high selenium diets of either selenium source.
Can the organic selenium deposited in pig tissue affect humans consuming these pork products?
The toxicosis effects in humans are not as easily discernible as in swine. In general, the signs of selenosis are similar to those demonstrated in the pig. There are some foods that are extremely high in selenium; and if they are consumed they can cause the selenosis condition. Palmer et al. (1982) analyzed Brazil nuts and found that they averaged 29.6 ppm Se, but approximately 6% of the nuts contained more than 100 ppm Se. From the field studies, it appears that an intake of 5 mg Se/day from an organic source (i.e., food) or approximately 1 mg Se/day from an inorganic source when consumed for an extended time period will produce hair loss, changes in fingernails, and an odorous smell in the breath (Table 8). Jensen et al. (1984) reported that 2.3 g of sodium selenite consumed for a 2.5 month period produced the classical selenosis conditions in humans.
If we use the selenium tissue data from Table 4 and the data from Mahan and Parrett (1996) where lower dietary selenium levels were fed to grower-finisher pigs, we can calculate the amount of pork meat that would need to be consumed daily to potentially cause the selenosis effect in humans (Table 9). This assumes that the pigs had been fed either the inorganic or organic selenium during their entire grower-finisher period. The selenium concentrations of the tissues from these studies were used as the basis for these calculations. The toxic level for the human is not clearly defined, but if we assume a lower level of 1 mg/day and that pigs would have consumed either inorganic or organic selenium at 0.5 ppm Se, the calculated results suggest that a daily intake of 7.1 kg of pork loin or 1.4 kg of pork liver would be needed to produce the toxic effects if the pig had been fed inorganic selenium. If however, the pig had been fed organic selenium, the amount of pork loin would be lower (3.8 kg/day) while liver would be 1.4 kg/day when consumed daily over at least a two year period. Quantities of both meats are in great excess of normal eating patterns.
Table 8. Selenium toxicosis in humans.
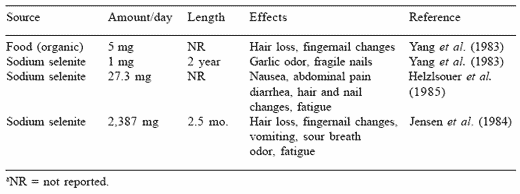
Table 9. Calculated amount (kg) of pork (loin or liver) needed to possibly produce toxicosis in humans (assuming 1 or 5 mg daily selenium consumption from the meat sources). ab
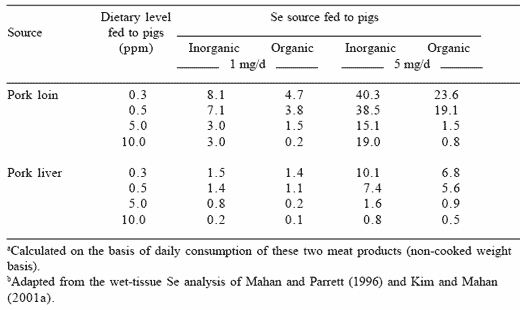
If however, we establish the toxic level at 5 mg/ day (which would be a more reasonable estimate) the daily amount of pork loin needed to produce a toxic effect would be 38.5 kg when inorganic selenium was fed to the pig at 0.5 ppm Se, or 19.1 kg/day if organic selenium were added to the diet. The amount of pork liver required would be 7.4 or 5.6 kg/day for the two sources, respectively. These results demonstrate that pigs consuming either inorganic or organic selenium at dietary levels #0.50 ppm Se will not result in meat levels that would produce the selenosis effects in humans. In fact, it is clear that much of the world is at low dietary selenium intakes and supplementation appears to be necessary to achieve the benefits of improved health (Rayman, 2000). The data of Clark et al. (1996) further indicate that the daily consumption of 0.4 mg Se/day over a 10-year period resulted in a lowered incidence of body cancers by approximately 50%.This suggests that selenium-enriched meat may be a valuable mechanism to enhance human health benefits and possibly the prevention of some of our most health threatening problems.
References
Anonymous. 1999. Poison? Miracle Nutrient? S. Dakota State University; Farm & Home Research. 50:4-9.
Clark, L.C., G.F. Combs, B.W. Turnbull, E.H. Slate, D.K. Chalker, J. Chow, L.S. Davis, R.A. Glover, G.F. Graham, E.G. Gross, A. Krongrad, J.l. Lesher, H.K. Park, B.B. Sanders, C. L. Smith and J.R. Taylor. 1996. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. J. Am. Med. Assoc. 276:1957-1963.
Diehl, D.S., D.C. Mahan and A.L. Moxon. 1975. Effects of single intramuscular injections of selenium at various levels to young swine. J. Anim. Sci. 40:844-850.
Ekermans, L. G., and J. V. Schneider. 1982. Selenium in livestock production: A review. J. South Afri. Vet. Assoc. 53:223-228.
Goehring T.B., I.S. Palmer, O.E. Olson, G.W. Libal and R.C. Wahlstrom. 1984a. Effects of seleniferous grains and inorganic selenium on tissue and blood composition and growth performance of rats and swine. J. Anim. Sci. 59:725-732.
Goehring T.B., I.S. Palmer, O.E. Olson, G.W. Libal and R.C. Wahlstrom. 1984b. Toxic effects of selenium on growing swine fed corn-soybean meal diets. J. Anim. Sci. 59:733-737.
Helzlsouer, K., R. Jacobs and S. Morris. 1985. Acute selenium intoxication in the United States. Fed. Proc. 44:1670 (Abstr.).
Herigstad, R.R. C.K. Whitehair and O.E. Olson. 1973. Inorganic and organic selenium toxicosis in young swine: Comparison of pathologic changes with those in swine with vitamin E-selenium deficiency. Am. J. Vet. Res. 34:1227-1238.
Jensen, R., W. Closson and R. Rothenberg. 1984. Selenium intoxication – New York. Morb. Mortal. Week. Rep. 33:157-158.
Kim, Y.Y. and D.C. Mahan. 2001a. Comparative effects of high dietary levels of organic and inorganic selenium on selenium toxicity of grower finisher pigs. J. Anim. Sci. (in press).
Kim, Y.Y. and D.C. Mahan. 2001b. Prolonged feeding of high dietary levels of organic and inorganic selenium to gilts from 25 kg body weight through one parity. J. Anim. Sci. (in press).
Ku, P.K., W.T. Ely, A.W. Groce and D.E. Ullrey. 1972. Natural dietary selenium, "-tocopherol and effect on tissue selenium. J. Anim. Sci. 34:208-211.
Mahan, D.C., and A.L. Moxon. 1984. Effect of inorganic selenium supplementation on selenosis in postweaning swine. J. Anim. Sci. 58:1216-1221.
Mahan, D.C. and N.A. Parrett. 1996. Evaluating the efficacy of selenium enriched yeast and sodium selenite on tissue selenium retention and serum glutathione peroxidase activity in grower and finisher swine. J. Anim. Sci. 74:2967-2974.
Miller, W.T. and H.W. Schoening. 1938. Toxicity of selenium fed to swine in the form of sodium selenite. J. Agr. Res. 56:831-842.
Miller, W.T. and K.T. Williams. 1940. Minimum lethal dose of selenium, as sodium selenite, for horses, mules, cattle and swine. J. Agr. Res. 60:163-173.
Moxon, A.L. 1937. Alkali disease or selenium poisoning. In: Agricultural Experiment Station Bulletin, South Dakota State College of Agriculture and Mechanic Arts. Brookings, South Dakota. Bulletin 311, pp 3-87.
Oldfield, J.E. 1990. Selenium its uses in Agriculture•Nutrition•Health•Environment. Selenium-Tellurium Development Association, Inc. pp 8.
Olson. O.E., E.J. Novacek, E.I. Whitehead and I.S. Palmer. 1970. Investigation on selenium in wheat. Photochemistry. 9:1181-1188.
Palmer, I.S., A. Herr and T. Nelson. 1982. Toxicity of selenium in Brazil nuts to rats. J. Food Sci. 47:1595-1597.
Rayman, M.P. 2000. The importance of selenium to human health. Lancet 356:233-241.
Wolffram, S. 1999. Absorption and metabolism of selenium: differences between inorganic and organic sources. In: Biotechnology in the Feed Industry, Proceedings of the 15th Annual Symposium (T.P. Lyons and K.A. Jacques, eds) Nottingham University Press, UK
Yang, G., S. Wang, R. Zhou and S. Sun. 1983. Endemic selenium intoxication of humans in China. Am. J. Clin. Nutr. 37:872-881.
Related topics
Join to be able to comment.
Once you join Engormix, you will be able to participate in all content and forums.
* Required information
Would you like to discuss another topic? Create a new post to engage with experts in the community.
Create a post




.jpg&w=3840&q=75)