The disease
African swine fever (ASF) is one of the most important infectious disease of swine. This disease causes greater sanitary, social and economic impacts due to its high mortality rate in domestic pigs and restrictions on the pig and pork trade. It is not a zoonotic disease, which limits its impact on public health. ASF must be notified to the World Organization of Animal Health (OIE). There is not a commercial vaccine or treatment against ASF at present, because of that, its control is based on early detection and the application of strict sanitary measures and of biosecurity.
The African swine fever virus (ASFV) is a complex, large, enveloped DNA virus with icosahedral morphology. It is classified as the only member of the Asfarviridae family, genus Asfivirus. It presents a huge genetic and antigenic variability existing 24 genotypes based on differences observed in the C-terminal region of the gene vp72. Genotype II is circulating in Eastern Europe since 2007, in the European Union (EU) since 2014 and in Asia since 2018
ASFV can infect all members of the suidae family. Domestic pig (Sus scrofa domestica) and wild boar (Sus scrofa) show a variety of clinical presentations: peracute, acute, subacute, chronic or subclinical (Sánchez-Vizcaíno & Arias, 2012). In Africa, wild suid species including warthogs (Phacochoerus spp.), bushpigs (Potamochoerus spp.) and the giant forest hog (Hylochoerus meinertzhageni), develop asymptomatic infections and act as ASFV reservoirs. ASFV also replicates in soft ticks of the Ornithodoros genus (Ornithodoros moubata in Africa and O. erraticus in the Iberian Peninsula), which act as biological vectors and reservoirs (Jori et al., 2013)
The transmission of the disease can occur through direct contact with secretions or excretions of infected animals, such as blood or feces. There may also be an indirect transmission through ingestion of meat or meat products from infected animals or through contaminated feed or fomites (e.g., swill feeding, vehicles, clothing, boots) (Sánchez-Vizcaíno et al., 2015). Finally, its transmission through the biological vectors (soft ticks of the genus Ornithodoros) or mechanical vectors (flies of the species Stomoxys calcitrans). (Mur et al., 2017)
The clinical signs vary depending on the species affected, the virulence of the virus, the infective dose and the route of infection among other factors. The genotype II that currently circulates in Europe and Asia is highly virulent and induces an acute form of the disease in domestic pig (Gabriel et al., 2011)
The acute form is characterized by the appearance of signs such as high fever, anorexia, erythema, hemorrhages in skin and internal organs, weakness, abortions, vomiting or diarrhea In these cases the mortality is close to 100% and usually occurs between 4 and 9 days after infection (Sánchez-Vizcaíno et al., 2015). However, the existence of wild boar survivors of genotype II infection, has play a leading role in the epidemiology of ASF in Europe, being involved in the maintenance of the disease in endemic areas (Armenia, Azerbaijan, Georgia, Russian Federation, Belarus and Ukraine) and in its introduction and dissemination within the countries of the European Union. Other genotypes of a lower virulence may give subacute, chronic or inapparent clinical forms, in which mortality rates are lower and recovered animals can act as potential carriers of the virus.
The diagnosis must always be confirmed with laboratory tests in order to perform a differential diagnosis with bacterial sepsis or other hemorrhagic diseases such as classical swine fever (CSF) that have similar clinical conditions. All techniques currently used to diagnose ASF have been fully validated, showing high sensitivity and specificity to detect antigens and antibodies against known genotypes, allowing the correct diagnosis of ASF in all possible epidemiological scenarios (Sánchez-Vizcaíno & Mur, 2013)
There is currently no commercialized vaccine or treatment for ASF. The measurements of prevention and control are based on early identification and diagnosis of the disease followed by the establishment of strict sanitary measures, so the development of an effective and safe vaccine is one of the most promising bets for control and PPA eradication (Arias et al., 2018)
Brief history of the disease
The ASF was discovered by Montgomery in Kenya in 1921, as an acute hemorrhagic fever what caused high mortality in imported European pigs. Since then, the disease has been reported in most sub-Saharan African Countries. The disease was entirely restricted to the African continent until 1957, when there was a first transcontinental spread to Portugal, near Lisbon, with an outbreak with mortalities close to 100%. The disease was rapidly controlled, however, after an epidemiological silence of three years, in 1960 the disease was notified again in Portugal, spreading in the following years throughout the Iberian Peninsula, several European countries (France, Italy, Malta, Belgium and Holland) and Latin American countries (Cuba, Brazil, Dominican Republic and Haiti). However, all these foci were eradicated except for the island of Sardinia where the disease remained endemic until 2019.
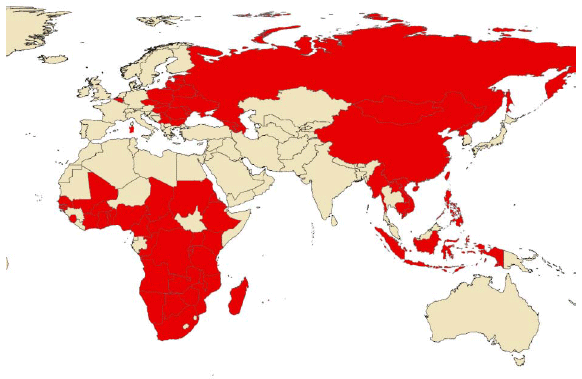
The years following its eradication were of apparent calm for Europe while the disease continued to spread and becoming increasingly important in Africa, affecting many countries previously free of the disease. This expansion facilitated a new spread to the European continent, in 2007, specifically in the Georgian port of Poti, through contaminated meat residues transported in ships from Africa and used to pig feed. All the control measures implemented below were unable to stop its spread and the disease began its unstoppable expansion across the European continent with a slow but steady geographical advance. The disease already affects 18 countries of the European continent (Georgia, Armenia, Russia, Azerbaijan, Ukraine, Belarus, Lithuania, Latvia, Estonia, Poland, Moldova, Czech Republic, Belgium, Bulgaria, Hungary, Romania, Slovakia and Serbia), 11 of them belonging to the European Union. In 2018, the virus reached the Asian continent for the first time in history, where it has spread rapidly and unstoppably, currently affecting 12 countries (China, Mongolia, Vietnam, Cambodia, North Korea, Laos, Slovakia, Myanmar, Philippines, South Korea, East Timor, Indonesia), (OIE, 2020).
Figure 1. Countries affected by African Swine Fever (OIE, 2020). Update in January 2020.
Current epidemiological scenarios
Africa. The African continent gives its name to the disease since it was where it was first described in 1921 by Montgomery and since then, the disease has been reported and remained endemic in many countries. In Africa, two different epidemiological scenarios are described.
The first one occurs in southeast Africa, where the disease is endemic. There is a very complex epidemiological cycle characterized by coexistence of wild suid species such as warthogs (Phacochoerus spp.), bush pigs (Potamochoerus spp.) and giant forest hogs (Hylochoerus meinertzhageni), Ornithodoros moubata soft ticks and domestic pigs. The wild suidae are distributed in abundant way in Southeast Africa and have the peculiarity of being tolerant to the ASF virus, since they are able to maintain very low blood virus levels that are not enough for the development of the disease, in such a way that they act as asymptomatic carriers of ASF and reservoir of the virus. Also the virus infects and replicates in soft ticks of the genus Ornithorodoros Moubata which are the only known natural arthropod hosts of the virus and act as reservoirs and biological vectors in the transmission between wild suidae and domestic pigs. In this way, the three cycles of transmission have been confirmed in this region: two cycles that involve soft ticks (wild suid-tick and domestic pig-tick) and a cycle involving circulation among domestic pigs. In this area the 24 genotypes described to date have been identified.
The second scenario is less complex and one occurs in countries in west and central Africa. Although many countries in this area are at present endemically infected, the history of ASF in that region is more recent and likely due to introductions that occurred mainly in the late 1950s and again in the 1990s. To date only genotype I has been recovered in this area. In this epidemiological cycle, only domestic pigs participate and the transmission is due to contact between infected and susceptible animals, as well as the consumption of contaminated pig products (swill feeding). In the center of the African continent, specifically in Malawi, the transmission of the virus in domestic pigs through Ornithodoros porcinus has also been described.
Europe. In Europe, the disease affects both domestic pig and wild boar. However, the high density of wild boar, which play a fundamental role in the spread and maintenance of the virus acting as reservoirs and as a source of introduction of the disease through connected natural landscapes and spreading the disease by means of contact with domestic pigs.
As we have explained above, it is not the first time that the disease affects the European continent. When the disease affected Portugal and Spain, an epidemiological cycle was observed with soft ticks of the genus Ornithodoros of the species O. erraticus. However, the presence of these ticks has not been demonstrated in the countries currently affected and therefore their involvement in the current epidemiology could be nil.
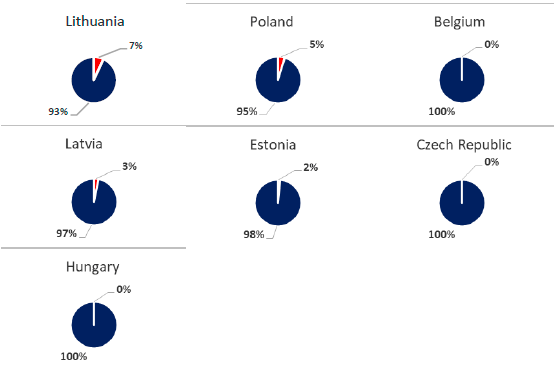
At present we can distinguish three epidemiological scenarios in Europe in function of the host affected predominantly. The first one occurs in the countries belonging to the European Union affected (except Romania, Bulgaria and Slovakia), where more than 90% of ASF cases in Europe are attributed to wild boar with sporadic outbreaks in domestic pig farms. In these countries, control strategies should be aimed at controlling wild boar populations and increasing biosecurity in domestic pig farms to avoid contact with wild boars.
Figure 2. Percentage of host affected by country, differentiating between domestic pig (red) and wild boar (blue), (OIE, 2020). Update in January 2020.
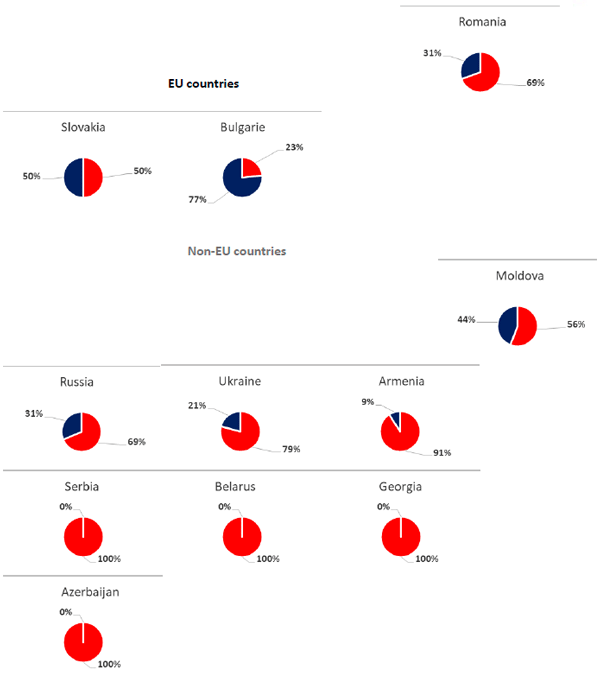
The second epidemiological scenario occurs in the countries of Eastern Europe (Armenia, Azerbaijan, Belarus, Georgia, Moldova, Serbia, Russia and Ukraine), Bulgaria, Romania and Slovakia. 40% of the reported cases are in wild boar and 60% in domestic pig. One of the main differences in this epidemiological scenario is the existence of a large number of family or backyard farms with poor levels of biosecurity, which are usually located in areas with wild boar, increasing the risk of virus transmission. In addition, in many of them, the animals are fed with contaminated food scraps. This practice is known as "swill feeding" and is currently prohibited in the European Union since it is an important route of disease transmission.
Figure 3. Percentage of host affected by country, differentiating between domestic pig (red) and wild boar (blue), (OIE, 2020). Update in January 2020.
The last epidemiological scenario in Europe is located in the Italian island of Sardinia, where the disease has been endemic since 1978. The endemic factors appear to be poor biosecurity conditions in farms, high densities of wild boar and local and, specially, traditional farming practices such as raising non-registered domestic pigs (free-ranging pigs). These free-ranging pigs, known as “brado”, are a virus reservoir and provide a route of transmission between domestic pigs kept in backyards and wild boar populations. (Jurado et al., 2018). Several control and eradicaton programmes, which included the prohibition of brado in 2012, have achieved to reduce the outbreaks and that there is no cases of ASF on the island during the year 2019.
Figure 4. Example of a camera trapping image showing direct interaction between a free-ranging pig and wild boar. (Cadenas-Fernández et al., 2019)

Asia. The Chinese authorities reported the first outbreak of ASF in Asia in August 2018, at a domestic pig farm located in Shenyang city. This was a spectacular breakthrough for the disease since China is the world's leading producer of pork, with about half of the pig population worldwide. Epidemiological studies indicated that the outbreak could have been due to the importation of infected piglets or “swill feeding”, common in Asian backyard farms. In 2019, the disease has spread considerably faster than in other epidemiological scenarios and 11 countries of the Asian continent are currently affected (China, Mongolia, Vietnam, Cambodia, North Korea, Laos, Myanmar, Philippines, South Korea, Timor-Leste and Indonesia). In these countries, 100% reported cases are in domestic pigs and the main causes are poor biosecurity measures applied on farms and "swill feeding". In addition, the notification of outbreaks in very separate places suggests the transport of infected animals or pork is facilitating this rapid spread. Therefore, control strategies in this continent should be focus on improving biosecurity and on the prohibition of "swill feeding”. The lack of compensation to the affected farmers is another important risk factor for the control of the disease as well as the partial depopulation carrier out in some farms.
Another notable aspect to consider in the Asian continent is the estimated high density of wild boars in some areas, even higher than in the European continent. This aspect is of great interest, since as in the European scenario, the wild boar could play a very important role in the endemism and maintenance of the ASF in Asia. So far, outbreaks reported in wild boars are very low compared to domestic pig, with the majority being concentrated in South Korea, affected since September 2019.
Current situation
The current situation of African swine fever has acquired a pandemic dimension and suggests an imminent risk for world swine production. The disease has spread to 47 countries on three continents. Currently, 77% of total swine population is already living in an infected area. China, the main pig producer, with about half the head of pigs from around the world, has lost more than 37% of its porcine population. Globalization has increased the risk of ASF being introduced into free areas. The main risks of ASF spread are the continuous movement of infected wild boar populations, the poor levels of biosecurity in farms and the illegal movement of live pigs and infected pig meat and products coming from infected areas.
A point to highlight in the control is the human factor, main responsible for the spread of the virus to new regions, with practices such as feeding of pigs with contaminated products (“swill feeding”), movement of infected pigs or products and in general, the lack of biosecurity measures. Moreover, we must pay close attention to wild boars. The density of this animal in Europe and Asia is very high and this host is very susceptible to virus infection. In this way, the wild boar is playing a very important role in the maintenance and endemism of the disease, as observed in Europe.
The control of this disease is very complex and requires a great effort at all levels (better communication about the disease, field-administration-research). One of the great challenges is the lack of treatment or commercial vaccine. At this point, vaccination is considered to be the most efficient strategy and solution for emerging infectious diseases. Regrettably, attempts over many years to develop a vaccine have failed. Last year new several vaccines prototypes for domestic pig and wild boar have been described with very promising results (Barasona et al., 2019).
In this critical situation, A EU project, “VACDIVA”, has been financed with 10 million euros by the European Union with the objective of develop an effective, safe and DIVA vaccine against ASF over the next four years (VACDIVA H2020 Grant ID: 862874). The research works involve two international ASF reference laboratories, both of them Spanish: the Centre for Veterinary Health Surveillance (VISAVET) of the University Complutense and the Animal Health Research Centre (CISA-INIA) of the National Institute for Agricultural and Food Research and Technology. Additionally, six national UE laboratories (from the 10 countries currently affected by ASF), four prestigious ASF research centres and two leader companies in vaccine production and ASF diagnostic kits. It also has the collaboration of Chinese, Russian and African laboratories, which will provide very useful support for the project. Finally, the active participation of pig producers, agricultural associations, hunting associations and international agencies, will expand the impact of the activities of communication, dissemination and training of the project.
The project, which will last the following four years, have three objectives:
- To provide an effective, safe and DIVA vaccine(s) for wild boar and domestic pigs already for registration.
- To develop DIVA test to allow an accurate monitoring of the effectiveness of the vaccine.
- To design ASF control an eradication strategies in different epidemiological scenarios worldwide and test the pilot vaccine in real environments (including bushpigs and warthogs).
Finally, the situation described is affecting very much the global swine industry, meat prices are increasing around the world and the pig production is trying to address these changes. A personal view of these future changes will be also discussed in this presentation.
Published in the proceedings of the International Pig Veterinary Society Congress – IPVS2020. For information on the event, past and future editions, check out https://ipvs2022.com/en.