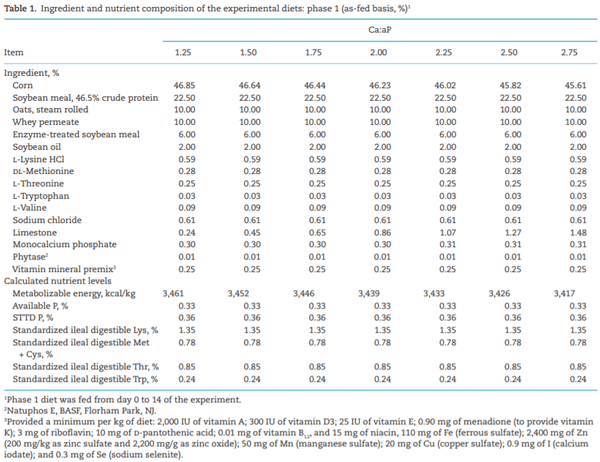
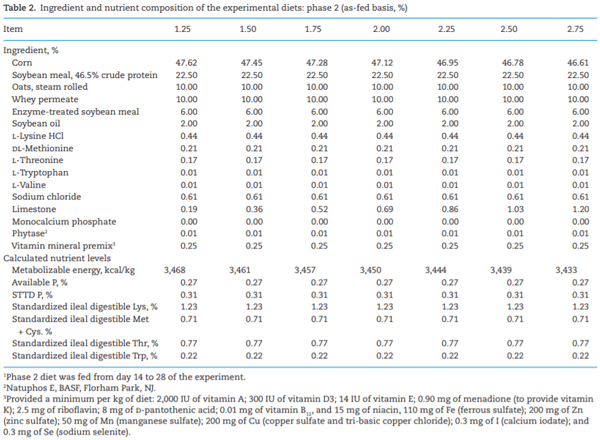
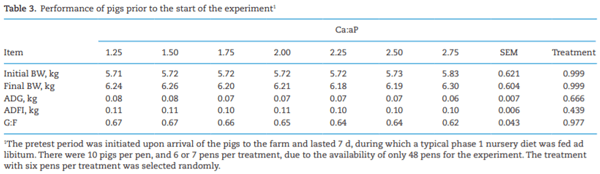
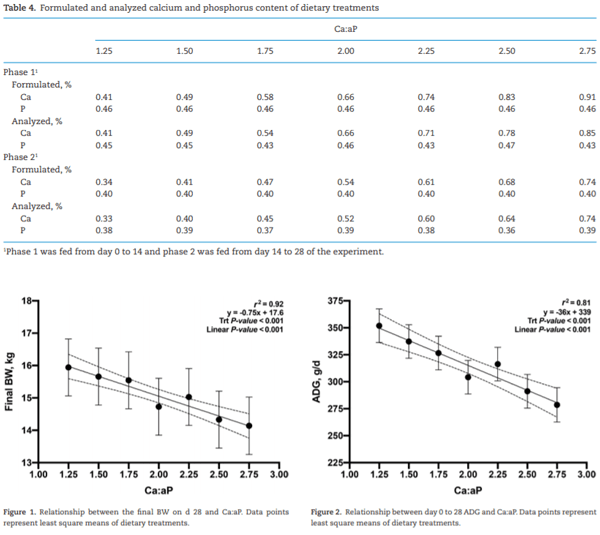
Adverse effects on growth performance and bone development in nursery pigs fed diets marginally deficient in phosphorus with increasing calcium to available phosphorus ratios
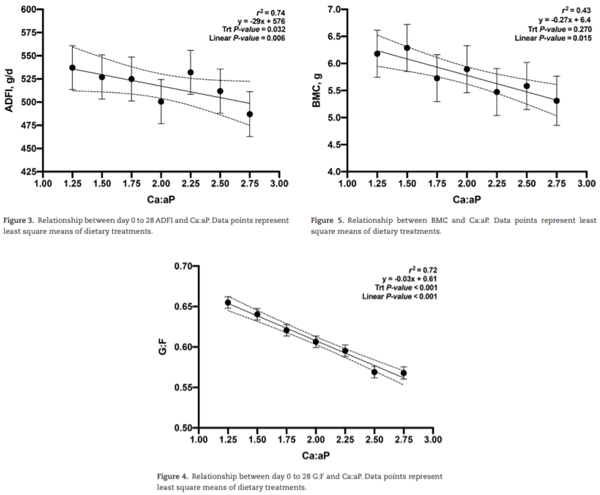
The objective of this experiment was to evaluate the growth performance and bone mineral content (BMC) of nursery pigs in response to increasing total calcium (Ca) to available phosphorus (aP) ratios in diets containing phytase (250 FTU/kg; Natuphos E, BASF, Florham Park, NJ). A total of 480 nursery pigs (body weight (BW) = 5.7 ± 0.6 kg) with 10 pigs per pen and 7 pens per treatment (6 pens fed 2.75:1 diet) were allotted to seven treatments consisting of increasing ratios of calcium to available phosphorus (Ca:aP): 1.25, 1.50, 1.75, 2.00, 2.25, 2.50, and 2.75. From day −7 to 0, pigs were fed a common diet. They were then fed the treatment diets during two experimental phases from day 1 to 14 and 15 to 28, respectively. Available P was formulated to 0.33% and 0.27% (approximately 90% of requirement) in dietary phases 1 and 2, respectively. BW, average daily gain (ADG), average daily feed intake (ADFI), and gain-to-feed ratio (G:F) were determined. BMC of the femur was measured on day 28 on one pig per pen using dual x-ray absorptiometry. Data were analyzed as a linear mixed model using PROC MIXED (SAS, 9.3). Orthogonal polynomial contrasts were used to determine the linear and quadratic effects of increasing the Ca:aP. Over the 28-d experimental period, increasing Ca:aP resulted in a linear decrease in ADG (353, 338, 328, 304, 317, 291, and 280 g/d; P < 0.01), ADFI (539, 528, 528, 500, 533, 512, and 489 g/d; P < 0.05), and G:F (0.68, 0.66, 0.64, 0.62, 0.61, 0.59, and 0.58; P < 0.01). Increasing Ca:aP also resulted in decreased BW on days 14 and 28 (P < 0.01). The BMC of the femur decreased with increasing Ca:aP (6.2, 6.3, 5.7, 5.9, 5.5, 5.6, and 5.3 g; P < 0.05). Regression analysis explained the impact of Ca:aP as follows on ADG (ADG [g/d] = 339 − 36x; r2 = 0.81), G:F (G:F = 0.61 – 0.03x; r2 = 0.72), and BMC (BMC [g] = 6.4 – 0.27x; r2 = 0.43), where x is the Ca:aP. In conclusion, all outcomes indicated that any level of calcium above the minimum used in this experiment impaired growth performance and skeletal development. Further research using even lower levels of dietary Ca is warranted.
Key words: bone mineral content, dual x-ray absorptiometry, swine
AAFCO. 2000. Official publication 2000. Atlanta (GA): Association of American Feed Control Officials Incorporated.
Akter, M. M., H. Graham, and P. A. Iji. 2018. Influence of different levels of calcium, non-phytate phosphorus and phytase on apparent metabolizable energy, nutrient utilization, plasma mineral concentration and digestive enzyme activities of broiler chickens. J. Appl. Anim. Res. 46:278–286. doi:10.1080/0 9712119.2017.1295972
Berndt, T., and R. Kumar. 2009. Novel mechanisms in the regulation of phosphorus homeostasis. Physiology (Bethesda). 24:17–25. doi:10.1152/physiol.00034.2008
Bridges, T. C., L. W. Turner, G. L. Cromwell, and J. L. Pierce. 1995. Modeling the effects of diet formulation on nitrogen and phosphorus excretion in swine waste. Appl. Eng. Agric. 11:731– 739. doi:10.13031/2013.25797
Crenshaw, T. D. 2001. Calcium, phosphorus, vitamin D, and vitamin K in swine nutrition. In: Lewis, A., and L. L. Southern, editors. Swine nutrition. 2nd ed. Boca Raton (FL): CRC Press; p. 196–221.
Cromwell, G. L. 2005. Phosphorus and swine nutrition. In: Sims, J. T., and A. N. Sharpley, editors. Phosphorus: agriculture and the environment. Madison (WI): ASA, CSSA & SSCA; p. 607–634.
Edixhoven, J. D., J. Gupta, and H. H. G. Savenije. 2013. Recent revisions of phosphate rock reserves and resources: reassuring or misleading? An in-depth literature review of global estimates of phosphate rock reserves and resources. Earth Syst. Dynam. Discuss. 4:1005–1034. doi:10.5194/ esdd-4-1005-2013
FASS. 2010. Guide for the care and use of agricultural animals in research and teaching. 3rd ed. Champaign (IL): Federation of Animal Science Societies.
Gonza´lez-Vega, J. C., Y. Liu, J. C. McCann, C. L. Walk, J. J. Loor, and H. H. Stein. 2016a. Requirement for digestible calcium by eleven- to twenty-five–kilogram pigs as determined by growth performance, bone ash concentration, calcium and phosphorus balances, and expression of genes involved in transport of calcium in intestinal and kidney cells. J. Anim. Sci. 94:3321. doi:10.2527/jas.2016-0444
González-Vega, J. C., C. L. Walk, M. R. Murphy, and H. H. Stein. 2016b. Requirement for digestible calcium by 25 to 50 kg pigs at different dietary concentrations of phosphorus as indicated by growth performance, bone ash concentration, and calcium and phosphorus balances. J. Anim. Sci. 94:5272– 5285. doi:10.2527/jas.2016-0751
Gutierrez, N. A., N. V. L. Serao, A. J. Elsbernd, S. Hansen, C. L. Walk, M. R. Bedford, and J. F. Patience. 2015. Quantitative relationships between standardized total tract digestible phosphorus and calcium intake and its retention and excretion in growing pigs fed corn-soybean meal diets. J. Anim. Sci. 93:2174–2182. doi:10.2527/jas.2014-8623
Gutzwiller, A., P. Schlegel, D. Guggisberg, and P. Stoll. 2014. Effects of benzoic acid and dietary calcium:phosphorus ratio on performance and mineral metabolism of weanling pigs. Asian-Australas. J. Anim. Sci. 27:530–536. doi:10.5713/ ajas.2013.13527
Hays, V. W. 1976. NFIA literature review on phosphorus in swine nutrition. West Des Moines (IA): National Feed Ingredient Association.
Heaney, R. P., and B. E. Nordin. 2002. Calcium effects on phosphorus absorption: implications for the prevention and co-therapy of osteoporosis. J. Am. Coll. Nutr. 21:239–244. doi:10 .1080/07315724.2002.10719216
Heyer, C. M., E. Weiss, S. Schmucker, M. Rodehutscord, L. E. Hoelzle, R. Mosenthin, and V. Stefanski. 2015. The impact of phosphorus on the immune system and the intestinal microbiota with special focus on the pig. Nutr. Res. Rev. 28:67– 82. doi:10.1017/S0954422415000049
Lagos, L. V., S. A. Lee, G. Fondevila, C. L. Walk, M. R. Murphy, J. J. Loor, and H. H. Stein. 2019a. Influence of the concentration of dietary digestible calcium on growth performance, bone mineralization, plasma calcium, and abundance of genes involved in intestinal absorption of calcium in pigs from 11 to 22 kg fed diets with different concentrations of digestible phosphorus. J. Anim. Sci. Biotech. 10:47. doi:10.1186/ s40104-019-0349-2
Lagos, L. V., C. L. Walk, M. R. Murphy, and H. H. Stein. 2019b. Effects of dietary digestible calcium on growth performance and bone ash concentration in 50- to 85-kg growing pigs fed diets with different concentrations of digestible phosphorus. Anim. Feed Sci. Technol. 247:262–272. doi:10.1016/j. anifeedsci.2018.11.019
Létourneau-Montminy, M. P., C. Jondreville, D. Sauvant, and A. Narcy. 2012. Meta-analysis of phosphorus utilization by growing pigs: effect of dietary phosphorus, calcium and exogenous phytase. Animal 6:1590–1600. doi:10.1017/ S1751731112000560
Létourneau-Montminy, M. P., A. Narcy, J. Y. Dourmad, T. D. Crenshaw, and C. Pomar. 2015. Modeling the metabolic fate of dietary phosphorus and calcium and the dynamics of body ash content in growing pigs. J. Anim. Sci. 93:1200–1217. doi:10.2527/jas.2014-8519
Levine, B. S., K. Ho, K. Kurokawa, and J. W. Coburn. 1984. Early renal adaptation to dietary phosphorus restriction. Miner Electrolyte Metab. 10: 222–227. doi:10.1007/978-1-4684-4808-5_15
Liu, J., D. W. Bollinger, D. R. Ledoux, and T. L. Veum. 1998. Lowering the dietary calcium to total phosphorus ratio increases phosphorus utilization in low-phosphorus corn-soybean meal diets supplemented with microbial phytase for growing-finishing pigs. J. Anim. Sci. 76:808–813. doi:10.2527/1998.763808x
Merriman, L. A., C. L. Walk, M. R. Murphy, C. M. Parsons, and H. H. Stein. 2017. Inclusion of excess dietary calcium in diets for 100- to 130-kg growing pigs reduces feed intake and daily gain if dietary phosphorus is at or below the requirement. J. Anim. Sci. 95:5439–5446. doi:10.2527/jas2017.1995
Misiura, M. M., J. A. N. Filipe, C. L. Walk, and I. Kyriazakis. 2020. How do pigs deal with dietary phosphorus deficiency? Br. J. Nutr. 124:256–272. doi:10.1017/S0007114520000975
Murer, H., N. Hernando, I. Forster, and J. Biber. 2003. Regulation of Na/Pi transporter in the proximal tubule. Annu. Rev. Physiol. 65:531–542. doi:10.1146/annurev.physiol.65.042902.092424
Nielson, A. J. 1972. Deposition of calcium and phosphorus in growing pigs determined by balance experiments and slaughter investigations. Acta. Agric. Scand. 22:223–237. doi:10.1080/00015127209433486
NRC. 2012. Nutrient requirements of swine. 11th rev. ed. Washington (DC): National Academies Press.
Olsen, K. M., S. A. Gould, C. L. Walk, N. V. L. Serao, S. L. Hansen, and J. F. Patience. 2019. Evaluating phosphorus release by phytase in diets fed to growing pigs that are not deficient in phosphorus. J. Anim. Sci. 97:327–337. doi:10.1093/jas/sky402
Oster, M., F. Just, K. Büsing, P. Wolf, C. Polley, B. Vollmar, E. Muráni, S. Ponsuksili, and K. Wimmers. 2016. Toward improved phosphorus efficiency in monogastrics-interplay of serum, minerals, bone, and immune system after divergent dietary phosphorus supply in swine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310:R917–R925. doi:10.1152/ajpregu.00215.2015
Patience, J. F. 2017. The theory and practice of feed formulation. In: Moughan, P., K de Lange, and W. Hendriks, editors. Feed evaluation science. Wageningen (The Netherlands): Wageningen Academic Press; p. 457–490.
Peo, E. R. Jr. 1976. NFIA literature review on calcium in swine nutrition. West Des Moines (IA): National Feed Ingredient Association.
Portale, A. A., B. P. Halloran, and R. Curtis. 1989. Physiological regulation of the serum concentration of 1,25-dihydroxyvitamin D by phosphorus in normal men. J. Clin. Invest. 83:1494–1499. doi:10.1172/JCI114043
Pravina, P., D. Sayaji, and M. Avinash. 2013. Calcium and its role in the human body. Int. J. Res. Pharmaceut. Biomed. Sci. 4:659–668.
Qian, H., E. T. Kornegay, and D. E. Conner Jr. 1996. Adverse effects of wide calcium:phosphorus ratios on supplemental phytase efficacy for weanling pigs fed two dietary phosphorus levels. J. Anim. Sci. 74:1288–1297. doi:10.2527/1996.7461288x
Reinhart, G. A., and D. C. Mahan. 1986. Effect of various calcium:phosphorus ratios at low and high dietary phosphorus for starter, grower and finishing swine. J. Anim. Sci. 63:457–466. doi:10.2527/jas1986.632457x
Saddoris, K. L., J. C. Fleet, and J. S. Radcliffe. 2010. Sodiumdependent phosphate uptake in the jejunum is posttranscriptionally regulated in pigs fed a low-phosphorus diet and is independent of dietary calcium concentration. J. Nutr. 140:731–736. doi:10.3945/jn.109.110080
Schlegel, P., and A. Gutzwiller. 2020. Dietary calcium to digestible phosphorus ratio for optimal growth performance and bone mineralization in growing and finishing pigs. Animals 10:178. doi:10.3390/ani10020178
Segawa, H., I. Kaneko, S. Tamanaka, M. Ito, M. Kuwahata, Y. Inoue, S. Kato, and K. Miyamoto. 2004. Intestinal Na-Pi cotransporter adaptation to dietary Pi content in vitamin D receptor null mice. Am. J. Physiol. Renal Physiol. 287:F39–47. doi:10.1152/ ajprenal.00375.2003
Stein, H. H., O. Adeola, G. L. Cromwell, S. W. Kim, D. C. Mahan, and P. S. Miller; North Central Coordinating Committee on Swine Nutrition (NCCC-42). 2011. Concentration of dietary calcium supplied by calcium carbonate does not affect the apparent total tract digestibility of calcium, but decreases digestibility of phosphorus by growing pigs. J. Anim. Sci. 89:2139–2144. doi:10.2527/jas.2010-3522
Wu, F., M. D. Tokach, S. S. Dritz, J. C. Woodworth, J. M. DeRouchey, R. D. Goodband, M. A. D. Gonçalves, and J. R. Bergstrom. 2018. Effects of dietary calcium to phosphorus ratio and addition of phytase on growth performance of nursery pigs. J. Anim. Sci. 96:1825–1837. doi:10.1093/jas/sky101










.jpg&w=3840&q=75)