In Vitro Activity of Neem (Azadirachta indica) Oil on Growth and Ochratoxin A Production by Aspergillus carbonarius Isolates
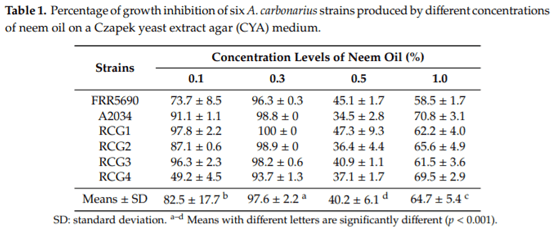
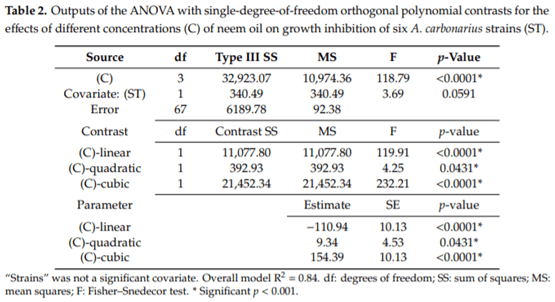
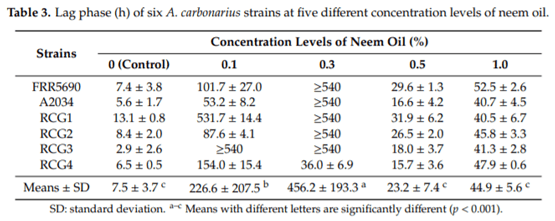
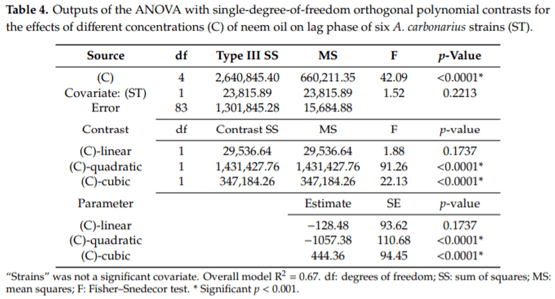
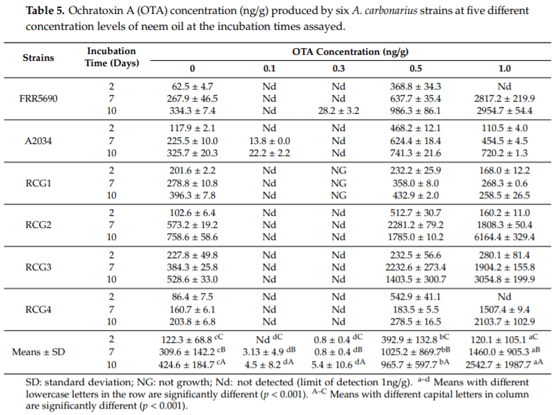
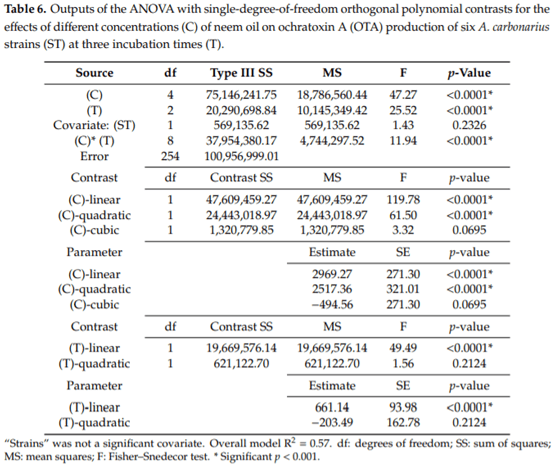
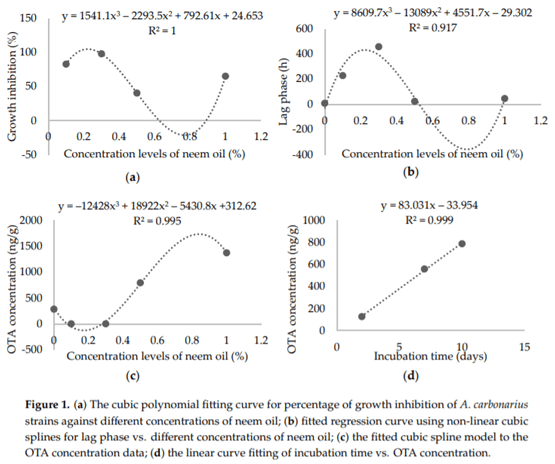
Aspergillus carbonarius is a saprobic filamentous fungus, food spoiling fungus and a producer of ochratoxin A (OTA) mycotoxin. In this study, the in vitro antifungal activity of neem oil (0.12% p/p of azadirachtin) was evaluated against the growth of six strains of A. carbonarius and the production of OTA. Four different concentrations of neem oil were tested in addition to three incubation times. Only the concentration of 0.3% of neem oil inhibited more than 95% of the strain’s growth (97.6% ± 0.5%), while the use of 0.5% and 1.0% of neem oil showed lower antifungal activity, 40.2% ± 3.1 and 64.7% ± 1.1, respectively. There was a complete inhibition of OTA production with 0.1% and 0.3% neem oil in the four strains isolated in the laboratory from grapes. The present study shows that neem essential oil can be further evaluated as an auxiliary method for the reduction of mycelial growth and OTA production.
Keywords: mycotoxins; essential oils; ecophysiology
Key Contribution: Neem oil was an effective inhibitor of mycelial growth of the assayed Aspergillus carbonarius strains and ochratoxin A production in vitro.

1. Pitt, J.I. Toxigenic fungi and mycotoxins. Br. Med. Bull. 2000, 56, 184–192. [CrossRef] [PubMed]
2. Gams, W.; Christensen, M.; Onions, A.H.S.; Pitt, J.I.; Samson, R.A. Infrageneric taxa of Aspergillus. In Advances in Penicillium and Aspergillus Systematics; Samson, R.A., Pitt, J.I., Eds.; Plenum: New York, NY, USA, 1985; pp. 55–61.
3. Varga, J.; Frisvad, J.C.; Kocsubé, S.; Brankovics, B.; Tóth, B.; Sziget, G.; Samson, R.A. New and revisited species in Aspergillus section Nigri. Stud. Mycol. 2011, 69, 1–17. [CrossRef] [PubMed]
4. Taniwaki, M.H.; Pitt, J.I.; Magan, N. Aspergillus species and mycotoxins: Occurrence and importance in major food commodities. Curr. Opin. Food Sci. 2018, 23, 38–43. [CrossRef]
5. Cabañes, F.J.; Bragulat, M.R. Black aspergilli and ochratoxin A-producing species in foods. Curr. Opin. Food Sci. 2018, 23, 1–10. [CrossRef]
6. CAST. Mycotoxins: Risks in Plant, Animal, and Human Systems, n. 139; CAST: Motor City, IA, USA, 2003; p. 199.
7. Tao, Y.; Xiea, S.; Xua, F.; Liub, A.; Wangb, Y.; Chenb, D.; Pana, Y.; Huangb, L.; Penga, D.; Wanga, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. 2018, 112, 320–331. [CrossRef] [PubMed]
8. IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, v. 56. 1993. Available online: https://monographs.iarc.fr/iarc-monographs-on-the-evaluation-of-carcinogenic-risks-to-humans-65/ (accessed on 4 April 2018).
9. Pfohl-Leszkowicz, A.; Petkova-Bocharova, T.; Chernozemsky, I.N.; Castegnaro, M. Balkan endemic nephropathy and associated urinary tract tumours: A review on aetiological causes and the potential role of mycotoxins. Food Addit. Contam. 2002, 19, 282–302. [CrossRef]
10. Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, 1231–1249. [CrossRef] [PubMed]
11. Calo, J.R.; Crandall, P.G.; O’Bryan, C.; Ricke, A.S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [CrossRef]
12. Yang, C.; Chowdhury, M.A.K.; Huo, Y.; Gong, J. Phytogenic Compounds as Alternatives to In-Feed Antibiotics: Potentials and Challenges in Application. Pathogens 2015, 4, 137–156. [CrossRef]
13. Stevanovi´c, Z.D.; Bošnjak-Neumüller, J.; Paji´c-Lijakovi´c, I.; Raj, J.; Vasiljevi´c, M. Essential Oils as Feed Additives-Future Perspectives. Molecules 2018, 23, 1717. [CrossRef]
14. Pawar, V.C.; Thaker, V.S. In vitro efficacy of 75 essential oils against Aspergillus niger. Mycoses 2007, 49, 316–323. [CrossRef] [PubMed]
15. El-Nagerabi, S.A.F.; Al-Bahry, S.N.; Elshafie, A.E.; AlHilali, S. Effect of Hibiscus sabdariffa extract and Nigella sativa oil on the growth and aflatoxin B1 production of Aspergillus flavus and Aspergillus parasiticus strains. Food Control 2012, 25, 59–63. [CrossRef]
16. Ferreira, F.D.; Kemmelmeier, C.; Arrotéia, C.C.; Costa, C.L.; Mallmann, C.A.; Janeiro, V.; Ferreira, F.M.; Mossini, S.A.; Silva, E.L.; Machinski, M., Jr. Inhibitory effect of the essential oil of Curcuma longa L. and curcumin on aflatoxin production by Aspergillus flavus Link. Food Chem. 2013, 136, 789–793. [CrossRef] [PubMed]
17. Passone, M.A.; Girardi, N.S.; Etcheverry, M. Antifungal and antiaflatoxigenic activity by vapor contact of three essential oils, and effects of environmental factors on their efficacy. LWT Food Sci. Technol. 2013, 53, 434–444. [CrossRef]
18. Manso, S.; Pezo, D.; Gómez-Lus, R.; Nerín, C. Diminution of aflatoxin B1 production caused by an active packaging containing cinnamon essential oil. Food Control 2014, 45, 101–108. [CrossRef]
19. Gupta, S.C.; Prasad, S.; Tyagi, A.K.; Kunnumakkara, A.B.; Aggarwal, B. Neem (Azadirachta indica): An Indian traditional panacea with modern molecular basis. Phytomedicine 2017, 34, 14–20. [CrossRef] [PubMed]
20. Quelemes, P.V.; Perfeito, M.L.G.; Guimarães, M.A.; dos Santos, R.C.; Lima, D.F.; Nascimento, C.; Silva, M.P.N.; Soares, M.J.S.; Ropke, C.D.; Eaton, P.; et al. Effect of neem (Azadirachta indica A. Juss) leaf extract on resistant Staphylococcus aureus biofilm formation and Schistosoma mansoni worms. J. Ethnopharmacol. 2015, 175, 287–294. [CrossRef]
21. Suresh, G.; Narasimhan, N.S.; Masilamani, S.; Partho, R.D.; Gopalakrishnan, G. Antifungal Fractions and Compounds from Uncrushed Green Leaves of Azadirachta indica. Phytoparasitica 1997, 25, 33–39. [CrossRef]
22. Siddiqui, B.S.; Afshan, F.; Ghiasuddin, S.F.; Naqvi, S.N.H.; Tariq, R.M. New insect-growth-regulator meliacin butenolides from the leaves of Azadirachta indica A. Juss. J. Chem. Soc. Perkin Trans. 1999, 16, 2367–2370. [CrossRef]
23. Van der Nat, J.M.; van der Sluis, W.G.; Hart, L.A.; Dijk, H.V.; de Silva, K.T.D.; Labadie, R.P. Activity-Guided Isolation and Identification of Azadirachta indica Bark Extract Constituents which Specifically Inhibit Chemiluminescence Production by Activated Human Polymorphonuclear Leukocytes. Planta Med. 1991, 57, 65–68. [CrossRef]
24. Govindachari, T.R.; Suresh, G.; Gopalakrishnan, G.; Banumathy, B.; Masilamani, S. Identification of Antifungal Compounds from the Seed Oil of Azadirachta indica. Phytoparasitica 1998, 26, 109–116. [CrossRef]
25. Roychoudhury, R. Chapter 18—Neem Products. In Ecofriendly Pest Management for Food Security; Omkar, Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 545–562. [CrossRef]
26. Brahmachari, G. Neem–An Omnipotent Plant: A Retrospection. ChemBioChem 2004, 5, 408–421. [CrossRef] [PubMed]
27. Del Serrone, P.; Failla, S.; Nicoletti, M. Natural control of bacteria affecting meat quality by a neem (Azadirachta indica A. Juss) cake extract. Nat. Prod. Res. 2015, 29, 985–987. [CrossRef] [PubMed]
28. Al Akeel, R.; Mateen, A.; Janardhan, K.; Gupta, V.C. Analysis of anti-bacterial and anti oxidative activity of Azadirachta indica bark using various solvents extracts. Saudi J. Biol. Sci. 2017, 24, 11–14. [CrossRef]
29. Al Saiqali, M.; Tangutur, A.D.; Banoth, C.; Bhukya, B. Antimicrobial and anticancer potential of low molecular weight polypeptides extracted and characterized from leaves of Azadirachta indica. Int. J. Biol. Macromol. 2018, 114, 906–921. [CrossRef]
30. Parida, M.M.; Upadhyay, C.; Pandya, G.; Jana, A.M. Inhibitory potential of neem (Azadirachta indica Juss) leaves on Dengue virus type-2 replication. J. Ethnopharmacol. 2002, 79, 273–278. [CrossRef]
31. Chaube, S.K.; Shrivastav, T.G.; Tiwari, M.; Prasad, S.; Tripathi, A.; Pandey, A.K. Neem (Azadirachta indica L.) leaf extract deteriorates oocyte quality by inducing ROS-mediated apoptosis in mammals. SpringerPlus 2014, 3, 464. [CrossRef]
32. European Commission. Available online: http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/ public/?event=activesubstance.ViewReview&id=721 (accessed on 13 August 2019).
33. Gowda, N.K.S.; Malathi, V.; Suganthi, R.U. Effect of some chemical and herbal compounds on growth of Aspergillus parasiticus and aflatoxin production. Anim. Feed Sci. Tech. 2004, 116, 281–291. [CrossRef]
34. Zeringue, H.J.; Bhatnagar, D. Effects of neem leaf volatiles on submerged cultures of aflatoxigenic Aspergillus parasiticus. Appl. Environ. Microbiol. 1994, 60, 3543–3547.
35. Razzaghi-Abyaneh, M.; Allameh, A.; Tiraihi, T.; Shams-Ghahfarokhi, M.; Ghorbanian, M. Morphological alterations in toxigenic Aspergillus parasiticus exposed to neem (Azadirachta indica) leaf and seed aqueous extracts. Mycopathologia 2005, 159, 565–570. [CrossRef]
36. Sitara, U.; Niaz, I.; Naseem, J. Antifungal effect of essential oils on in vitro growth of pathogenic fungi. Pak. J. Bot. 2008, 40, 409–414.
37. Bhatnagar, D.; McCormick, S.P. The Inhibitory Effect of Neem (Azadirachta indica) Leaf Extracts on Aflatoxin Synthesis in Aspergillus parasiticus. J. Am. Oil Chem. Soc. 1988, 65, 1166–1168. [CrossRef]
38. Zeringue, H.J.; Bhatnagar, D. Inhibition of Aflatoxin Production in Aspergillus flavus Infected Cotton Bolls After Treatment with Neem (Azadirachta indica) Leaf Extracts. J. Am. Oil Chem. Soc. 1990, 67, 215–216. [CrossRef]
39. Zeringue, H.J.; Shih, B.Y.; Bhatnagar, D. Effects of clarified neem oil on growth and aflatoxin B formation in submerged and plate cultures of aflatoxigenic Aspergillus spp. Phytoparasitica 2001, 29, 1–4. [CrossRef]
40. Garcia, R.P.; Garcia, M.I. Laboratory evaluation of neem derivatives against Aspergillus growth and aflatoxin formation. Philipp. Agric. Sci. 1990, 73, 333–342.
41. Bok, J.W.; Keller, N.P. LaeA, a Regulator of Secondary Metabolism in Aspergillus spp. Eukaryot. Cell 2004, 3, 527–535. [CrossRef] [PubMed]
42. Lind, A.L.; Smith, T.D.; Saterlee, T.; Calvo, A.M.; Rokas, A. Regulation of Secondary Metabolism by the Velvet Complex is Temperature-Responsive in Aspergillus. Genes Genomes Genet. 2016, 6, 4023–4033.
43. Roze, L.V.; Chanda, A.; Linz, J.E. Compartmentalization and molecular traffic in secondary metabolism: A new understanding of established cellular processes. Fungal Genet. Biol. 2011, 48, 35–48. [CrossRef]
44. Arroteia, C.C.; Kemmelmeier, C.; Junior, M.M. Effect of aqueous and oily extracts of Neem [Azadirachta indica A. Juss (Meliaceae)] on patulin production in apples contaminated with Penicillium expansum. Ciência Rural. 2007, 37, 1518–1523.
45. Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [CrossRef]
46. Allameh, A.; Razzaghi-abyane, M.; Shams, M.; Rezaee, M.B.; Jaimand, K. Effects of neem leaf extract on production of aflatoxins and activities of fatty acid synthetase, isocitrate dehydrogenase and glutathione S-transferase in Aspergillus parasiticus. Mycopathologia 2002, 154, 79–84. [CrossRef] [PubMed]
47. Zaika, L.L.; Buchanan, R.L. Review of compounds affecting the biosynthesis or bioregulation of aflatoxins. J. Food Prot. 1987, 50, 691–708. [CrossRef] [PubMed]
48. Magnoli, C.; Violante, M.; Combina, M.; Palacio, G.; Dalcero, A. Mycoflora and ochratoxin-producing strains of Aspergillus section Nigri in wine grapes in Argentina. Lett. Appl. Microbiol. 2004, 39, 326–331. [CrossRef] [PubMed]
49. Bragulat, M.R.; Abarca, M.L.; Cabañes, F.J. An easy screening method for fungi producing ochratoxin A in pure culture. Int. J. Food Microbiol. 2001, 71, 139–144. [CrossRef]
50. Scudamore, K.A.; MacDonald, S.J. A collaborative study of an HPLC method for determination of ochratoxin A in wheat using immunoaffinity column clean-up. Food Addit. Contam. 1998, 15, 401–410. [CrossRef] [PubMed]
51. Quinn, G.P.; Keough, M.J. (Eds.) Experimental Design Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002; pp. 1–537.










