A number of cereals and other crops that are incorporated into the human and animal feed are susceptible to fungal attack and mycotoxin production, either in the field (before and after harvest) or during storage, transport, processing and feeding whenever conditions are favourable. The fungi involved produce highly toxic compounds as secondary metabolites referred to as mycotoxins, which can cause a variety of ill effects in humans and animals, from allergic responses to immunosuppression and cancer. In many cases, these mycotoxins can be found together in food and feed and among these; aflatoxin is the most intensively studied; however, another very important mycotoxin is ochratoxin A (Sweeney and Dobson 1998). Ochratoxin A causes significant losses and reduction in the profitability of poultry industry due to its effects on performance and health (Agwane and Lonkar 2004). It causes a reduction in productive performance (growth rate, feed consumption, poorer feed conversion and increased mortality (Singh et al. 2015). Ochratoxin A is a mycotoxin that is receiving increased attention worldwide because of the hazard it poses to animal and human health. A live yeast, S. cerevisiae, was found to alleviate the adverse effects of mycotoxicosis in poultry (Stanley et al. 1993). S. cerevisiae has shown considerable binding ability with several commonly occurring mycotoxins (Devegowda et al. 1998), and is also found more effective as a low-inclusion binder to bind mycotoxins present in contaminated poultry feed when compared with other physical or chemical materials (Mahesh and Devegowda 1996). Furthermore, S. cerevisiae and cell wall components of S. cerevisiae have the ability to alleviate the adverse effects of the several combinations of mycotoxins present naturally in feed (Aravind et al. 2003). The objective of this investigation was to study the efficacy of S. cerevisiae to ameliorate ochratoxicosis in broiler chickens.
Materials and methods
Ochratoxin production: The lyophilised preparation of Aspergillus westerdijkiae NRRL 3147 was obtained from U.S. Department of Agriculture, Peoria, Illinois (USA). This lyophilised preparation was revived on potato dextrose agar medium and used for experimentation. Ochratoxin was produced as per the method described by Singh et al. (2013). Cracked maize (50 g) was taken in 250 ml conical flasks. The moisture content of substrate was adjusted to 35%. Thus, flasks were plugged with non-absorbent cotton and sealed with aluminium foil. The flasks were autoclaved for 20 min at 121°C and inoculated with 1-week old mycelium of Aspergillus westerdijkiae NRRL 3174. The inoculated flasks were incubated in a BOD incubator for 14 days. After removal from the incubator, the flasks were dried at 70°C and the ochratoxin assays were performed as per AOAC (1995).
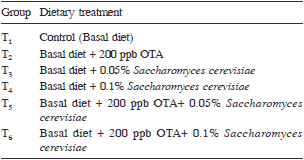
Experimental design: Experimental design was completely randomized design (CRD). There were 6 dietary treatments. Each dietary treatment had 5 replicates and each replicate had 8 chicks. The experiment was conducted in broiler chickens from day-old to 6 weeks of age. The various dietary treatments were prepared by mixing the required quantity of mouldy maize to get the desired concentration of 200 ppb OTA in basal diet (Table 1).
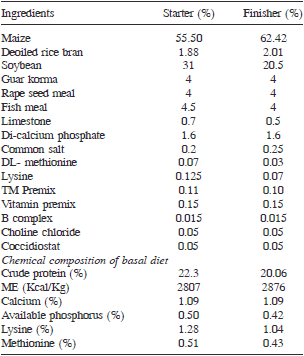
Biological experiment and analysis: Day-old broiler chicks (240) were obtained from experimental hatchery. The chicks were wing banded, weighed individually and distributed into 6 groups on equal weight basis. All birds were reared under standard managemental conditions from 0–6 weeks. All birds were fed with broiler starter ration from 1–21 days and broiler finisher ration from 22 to 42 days. Weekly individual body weight and feed consumption of each group were recorded. The ingredient and chemical composition of broiler starter and finisher ration are presented in Table 2.
The protein (AOAC 1995) and calcium (Talapatra et al. 1940) contents were estimated, while the concentrations of lysine, methionine, available P and metabolizable energy values were calculated. At the end of the experiment, 8 birds/ dietary treatment were sacrificed randomly and their organs were collected, weighed and expressed as percentage of live weight. The statistical analysis was done using SPSS 16.0 version.
Table 1. Experimental groups and treatments
Table 2. Ingredient and chemical composition of basal feed
Results and discussion
The data pertaining to body weight gain, feed intake and feed conversion ratio was statistically analysed and the average values are presented in Table 3.
Table 3. Body weight gain, feed intake and FCR in different growth phases as influenced by various dietary treatments.
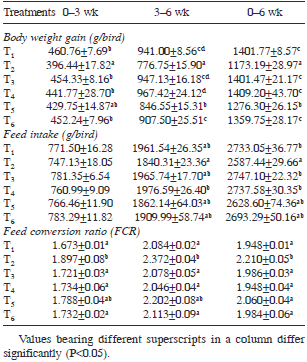
Body weight gain (BWG): Significant differences in BWG among dietary treatments were recorded from second week of age onwards. During starter phase (0–3 weeks) of growth trial, the BWG of broilers in ochratoxin alone fed group (T2) was lower (P<0.05) than that of control (T1). The BWG in other groups (T3 to T6) was statistically similar to that of control. During 3–6 weeks of growth trial, the BWG in ochratoxin alone fed group (T2) was lower (P<0.05) than that of control group (T1). The BWG in groups T3, T4 and T6 was statistically equal to that of control. The BWG in group T5 was higher (P<0.05) than that of T2 but lower than that of control group. During overall growth period (0–6 weeks), the BWG of birds in control group (T1) was higher (P<0.05) than that of ochratoxin alone fed group (T2). The BWG in groups T3 and T4 was statistically similar to that of control, suggesting that addition of S. cerevisiae to the basal feed did not produce any effect on BWG of birds. The overall BWG of group T5 was higher (P<0.05) than T2 but lower (P<0.05) than that of control (T1), indicating that addition of S. cerevisiae at 0.05% level partially ameliorated the adverse effects of ochratoxicosis on BWG of birds. The overall BWG in group T6 was higher (P<0.05) than T2 and statistically equal to that of control, indicating that addition of S. cerevisiae at 0.1% level to the ochratoxin contaminated feed ameliorated the adverse effects of ochratoxin on BWG in broiler chickens which resulted in welfare of birds.
The present study revealed that inclusion of 200 ppb ochratoxin in the diet of broiler chickens resulted in significant reduction in BWG of birds. Significant reduction in BWG of broiler chickens was in agreement with previous investigations with dietary ochratoxin levels of 50–100 ppb (Stoev et al. 2004, El-Barkouky 2008, EI-Barkouky and Abu-Taleb 2008) and 200 ppb (Sakhare et al. 2007, El- Barkouky et al. 2010 and Singh et al. 2015). In the present study, addition of 0.1% S. cerevisiae to the 200 ppb ochratoxin contaminated diet ameliorated the adverse effects of ochratoxin on weight gain in broiler chickens. El-Barkouky (2008) and El-Barkouky et al. (2010) reported that addition of S. cerevisiae to broiler diets provided partial protection against ill effects of ochratoxin on growth. Also, Mahesh and Devegowda (1996) found that S. cerevisiae has beneficial effects on weight gain of broiler chickens exposed to mycotoxins.
Feed intake (FI): During starter phase (0–3 weeks), the FI in various treatment groups did not differ significantly (P<0.05) from that of control. During finisher phase (3–6 weeks) of growth trial and overall growth period (0–6weeks), the FI in control group (T1) was higher (P<0.05) than that of ochratoxin alone fed group (T2). The feed intake in T3 and T4 was statistically similar to that of control, indicating that addition of S. cerevisiae to the basal feed did not produce any positive effect on feed intake of broilers. The FI in groups T5 and T6 was statistically similar to that of control, suggesting that addition of S. cerevisiae to the ochratoxin contaminated feed improved the feed consumption equal to that of control. In the present study, ochratoxin contamination in diet caused significant reduction in feed consumption of broiler chickens. Similarly, significantly reduced FI in broilers fed ochratoxin contaminated feed at a concentration ranging from 50–200 ppb, was observed by earlier workers (El-Barkouky 2008, El-Barkouky and Abu-Taleb 2008, El-Barkouky et al. 2010, Singh et al. 2015). The present study revealed that addition of S. cerevisiae to the ochratoxin contaminated feed ameliorated the adverse effects of ochratoxicosis on feed consumption of broiler chickens. El-Barkouky (2008) and El-Barkouky et al. (2010) also reported that addition of S. cerevisiae (3g/kg feed) to the 50–200 ppb ochratoxin contaminated diet improved the feed consumption in broiler chickens.
Feed conversion ratio (FCR): With respect to FCR in various growth phases, the FCR during starter phase (0–3 weeks) in control group (T1) was lower (P<0.05) than that of ochratoxin alone fed group (T2). The FCR in all other treatment groups (T3 to T6) was statistically similar to that of control. The FCR during 3–6 weeks in control group was lower (P<0.05) than that of ochratoxin alone fed group (T2). The FCR in other treatment groups (T3 to T6) was statistically (P<0.05) similar to that of control. In case of overall FCR (0–6 weeks), the FCR of ochratoxin alone fed group (T2) was higher (P<0.05) than that of control. The overall FCR in other treatment groups (T3 to T6) was statistically (P<0.05) similar to that of control. The present study revealed that ochratoxin contamination in feed increased (P<0.05) the FCR and resulted in poor feed efficiency in broiler chickens. These results are in agreement with earlier reports (Hanif et al. 2008, Sakhare et al. 2007, El-Barkouky 2008, El-Barkouky and Abu-Taleb 2008, El- Barkouky et al. 2010, Singh et al. 2015). In the present study, addition of S. cerevisiae (0.05% or 0.1%) to the 200 ppb ochratoxin contaminated feed ameliorated the adverse effects of ochratoxicosis on feed efficiency in broiler chickens. El-Barkouky (2008) and El-Barkouky et al. (2010) also reported that addition of active dried yeast S. cerevisiae at 3g per kg feed in the diet of broiler chickens ameliorated the ill effects of 50–200 ppb ochratoxin.
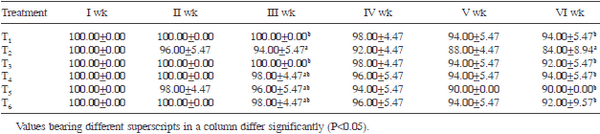
Table 4. Liveability percentage as influenced by various dietary treatments
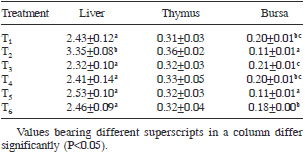
Table 5. Relative weight (% of live weight) of organs fed different dietary treatments
Livability percentage: The overall livability percentage (at sixth week of age) in control group (T1) was higher (P<0.05) than that of ochratoxin alone fed group (T2)(Table 4). The livability percentage of groups T3 and T4 was statistically similar to that of control. The livability percentage of groups T5 and T6 was higher (P<0.05) than that of T2 and statistically similar to that of control, suggesting that addition of S. cerevisiae to the ochratoxin contaminated diet improved the livability percentage by ameliorating the ill effects of ochratoxicosis in broiler chickens. In the present study, ochratoxin contamination of broiler diet caused higher mortality compared to control. These findings are in agreement with earlier reports in literature (El-Barkouky and Abu-Taleb 2008, El-Barkouky et al. 2010, Singh et al. 2015). The results of the present study revealed that addition of S. cerevisiae to the ochratoxin contaminated broiler diet reduced the mortality caused by ochratoxicosis, resulting in welfare of birds. Similar results were also reported by El-Barkouky (2008) and El-Barkouky et al. (2010).
Organ weights
Liver: The relative weight of liver (percent of live body weight) in control group (T1) was lower (P<0.05) than that of ochratoxin alone fed group (T2) (Table 5). The relative weight of liver in other treatment groups (T3 to T6) was statistically similar to that of control. In the present study, ochratoxin contamination in the diet of broiler chickens resulted in increased (P<0.05) relative weight of liver. Several researchers have also reported a significant increase in the relative weight of liver in broiler chickens (Elaroussi et al. 2008, Sakhare et al. 2007, El-Barkouky et al. 2010, Hanif et al. 2008, Singh et al. 2015) In the present study, supplementation of S. cerevisiae at both levels ameliorated the adverse effects of OTA on relative weight of liver. This result was in agreement with Baptista et al. (2002), who reported that dehydrated active yeast was able to reduce the hepatotoxicity caused by mycotoxins in hepatocytes.
Thymus: The relative weight of thymus in various dietary treatments did not vary significantly among treatments (Table 5). Inclusion of OTA to the basal diet did not result in any change in the relative weight of thymus. Addition of S. cerevisiae to the basal feed or OTA contamination feed did not bring any change in the relative weight of thymus in broiler chickens. This result is in agreement with earlier reports where no difference in the relative weight of thymus due to ochratoxin in feed was reported by Huff and Doerr (1981) and Hanif et al. (2008).
Bursa of Fabricius: The relative weight of bursa of Fabricius in control group (T1) was higher (P<0.05) than that of ochratoxin alone fed group (T2) (Table 5). The relative weight of bursa in T3 and T4 was statistically similar to that of control. In group T5, the relative weight of bursa was statistically similar to T2 and lower (P<0.05) than that of control, indicating that supplementation of S. cerevisiae at 0.05% level may not be sufficient to ameliorate adverse effect of ochratoxicosis on relative weight of bursa. The relative weight of bursa in group T6, was higher (P<0.05) than that of T2 and statistically similar to that of control, indicating that addition of S. cerevisiae (0.1%) ameliorated the ill effects of ochratoxin on relative weight of bursa. In the present study, ochratoxin contamination in feed resulted in significant (P<0.05) reduction in relative weight of bursa. Significant reduction in relative weight of bursa was earlier reported by Elarousi et al. (2008), Sakhare et al. (2007) and Singh et al. (2015). The present study showed that supplementation of 0.1% S. cerevisiae to the 200 ppb OTA contaminated diet resulted in significant improvement in the relative weight of bursa. This result is in agreement with El-Barkouky et al. (2010) who reported that addition of S. cerevisiae to the broiler diet provided protection against the adverse effects of OTA on relative weight of bursa.
It was concluded that ochratoxin contamination at 200 ppb level in broiler diet impaired the production performance in terms of body weight gain, feed intake, feed conversion efficiency, mortality and relative organ weights. Inclusion of Saccharomyces cerevisiae at 0.1% level to the ochratoxin contaminated diet ameliorated the ill effects of ochratoxicosis on production performance and relative weight of liver and bursa during 0–6 weeks of age in broiler chickens.
This article was originally published in Indian Journal of Animal Sciences 86 (7): 790–794, July 2016/Article.









.jpg&w=3840&q=75)