Understanding Mycotoxins And Their Impact On Animal Performance
The most commonly recognized feed-borne mycotoxins are the aflatoxins and the Fusarium mycotoxins. Our understanding of the etiology of aflatoxicosis is far more complete than our corresponding understanding of Fusarium mycotoxicoses. This is perhaps because of the large volume of literature fueled by the acutely carcinogenic nature of aflatoxins. Analytical methodology for aflatoxins in feedstuffs is also simple, sensitive and reproducible. This is due, in part, to the natural fluorescence of aflatoxin molecules.
Even though Fusarium mycotoxins are likely the most economically significant grain mycotoxins (Wood, 1992), the complex mixture of chemically-unrelated compounds has slowed advances in the study of Fusarium mycotoxicoses. Complete analysis of feedstuffs for Fusarium mycotoxins is a daunting, time-consuming and prohibitively expensive task.
It is also now clear that toxicological synergism between different Fusarium mycotoxins can increase the toxicity of a given diet (Smith et al., 1997) and incomplete analysis can, therefore, give false security as to the potential hazzard posed by the feeding of contaminated grains. It is important, also, to remember that many different components of a complete feed can be vectors for mycotoxin contamination.
The studies of Smith and Sousadias (1993, Table 1) indicated that the fusaric acid content of complete feeds can exceed that found in individual feedstuffs. This has since been attributed to fusaric acid contamination of soybean meal. Fusaric acid has been found in soybean plants and considered to be a phytotoxin in various vegetable species (Matsui and Watanabe, 1988).
Since almost all strains of Fusarium fungi produce fusaric acid, it has been suggested that this compound be used as a marker compound for Fusarium contamination of grains (Bacon et al., 1996). It is important, therefore, that complete feeds, and not just suspect individual ingredients, be analyzed when mycotoxin contamination is suspected.
Several trends have tended to increase the severity and economic importance of mycotoxicoses in animal and poultry agriculture in recent years. One key factor in minimizing fungal infestation in field crops and mycotoxin accumulation in feedstuffs is moisture content during the growing and harvesting periods. Stored grains should contain less that 15% moisture to ensure fungal stability.
Recent global weather patters, however, have been irregular with heavy rainfall and flooding in some areas coupled with drought and unusual frosts in other regions. Drought stress can also lead to increased fungal penetration of grains. The result has been an increased frequency of reports of mycotoxin contamination of feed grains. Some tropical and semi-tropical countries are reporting Fusarium contamination of crops where only aflatoxin was previously detected.
It is not clear, however, if this is also the result of increasingly frequent testing for compounds that were erroneously assumed to be absent in the past.
Another trend contributing to the frequency of mycotoxin contamination of feeds is improved global grain transportation systems and global trading of agricultural commodities. This allows more extensive shipping of grains and other feed components throughout the world. The result is that complete feeds are likely a more complex blend of feedstuffs with more widely varying geographic origins than was seen in the past. The potential for aflatoxins and mixtures of Fusarium mycotoxins to be cocontaminants in feeds is, therefore, enhanced.
The concept of mycotoxin binders
Mycotoxin binders are large molecular weight polymers that, when added to feed, are capable of forming irreversible complexes with mycotoxin molecules in the intestinal lumen. Such complexes are not digestible, pass down the digestive tract and are excreted in the feces.
The net effect is to reduce the dose of absorbed toxin to the point that it is below the biological threshold. This allows contaminated feed to be fed with minimal losses in performance. The challenge is to identify binders that are capable of effectively binding a mixture of mycotoxins with widely varying molecular structures and polarities.
The binder must also be effective at low levels of inclusion since these non-nutritive additives are diluents which will reduce the nutrient density of the diet. An example of a toxin binder that has been widely used in veterinary medicine to treat accidental acute poisonings is activated charcoal.
Mycotoxin binders can be silica-based inorganic polymers or carbon-based organic polymers. The inorganic polymers currently on the market include natural clay products as well as synthetic polymers. The advantage of the clay-based products is their low price. Unfortunately these products also offer low specificity and so must be used at a relatively high level of inclusion to be effective.
We found this to be the case for both bentonite (Carson and Smith, 1983a) and spent canola oil bleaching clays (Smith, 1984) when overcoming T-2 toxicosis. Synthetic inorganic polymers have usually be designed to effectively bind one specific mycotoxin. This is usually aflatoxin. Such specific products are, therefore, much less effective against a mixture of mycotoxins of varying molecular weight and polarity. Synthetic products are also inevitably more expensive than naturally-produced materials.
Organic toxin binders are derived from plant or microorganism fibres. Studies in our laboratory indicated that lignin-rich alfalfa fibre was quite effective at overcoming the toxicity of T-2 toxin (Carson and Smith, 1984b) and zearalenone (James and Smith, 1982; Stangroom and Smith, 1984). An advantage of using organic fibres as mycotoxin binders is that feedstuffs such as dehydrated alfalfa meal have some dietary energy and protein content and do not act as dietary diluents in the manner of inorganic polymers.
Alfalfa fibre, however, like clay-based products, is an effective mycotoxin binder at only high levels of dietary inclusion. This makes such materials impractical when added to livestock and poultry diets.
A innovation in mycotoxin binders is the concept of organic polymers derived from yeast cell wall. This material has a high surface area and enough specificity to allow effective myctoxin binding at a low level of dietary inclusion. This is the basis of the product Mycosorb (MTB-100) which is produced and marketed by Alltech, Inc.. A series of experiments was conducted, therefore to determine the effectiveness of yeast cell wall polymers in overcoming Fusarium mycotoxicoses in broiler chickens and swine.
Feeding trials with broiler chickens
A commercial strain of broiler chicks (Cobb, Big Four Hatchery) were fed soybean meal, corn and wheat-based diets for eight weeks at the Arkell Poultry Research Station of the University of Guelph. The diets included: (1) control (2) same diet formulated with a low level of contaminated corn and wheat (3) a diet containing higher levels of contaminated corn and wheat (4) the highly contaminated diet + 0.2% Mycosorb.
Three replicate pens of 30 birds were fed each diet. Weight gain and feed consumption were determined weekly. Diets were adjusted for protein levels corresponding to starter (0-3 weeks), grower (4-6 weeks) and finisher (7-8 weeks) phases. Diets were analyzed for deoxynivalenol, fusaric acid, zearalenone and T-2 toxin. Blood samples were taken at the end of the starter and finisher phases and a clinical screen of serum metabolites was conducted. At the end on the experiment, samples of breast, thigh and leg tissue were tested for discoloration using a Minolta colorimeter.
Results of the trial are given in Tables 2 and 3. Growth rate, feed consumption, feed efficiency and serum parameters were largely unaffected by diet with the exception of the finisher phase. In the finisher phase, the feeding of increasing levels of contaminated grains caused a significant depression in growth rates. This effect was overcome by the feeding of 0.2% Mycosorb.
It was also observed that blood RBC counts and concentrations of hemoglobin and uric acid increased with the feeding of increasing amounts of contaminated grains. An increasing redness of breast meat was also observed, as has been reported for turkey poults fed Fusarium culture material (Wu et al., 1994). The discoloration may be due to the effect of fusaric acid which acts as a hypotensive agent. It was concluded that Fusarium mycotoxicoses can be observed in broiler chickens with the feeding of naturally-contaminated grains. Such toxicoses can be reversed with the feeding of 0.2% Mycosorb.
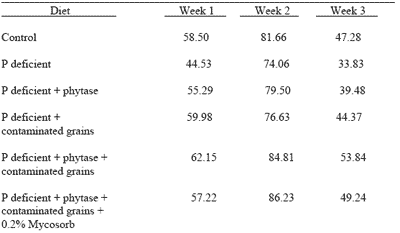
A study of the effect of Fusarium-contaminated grains on phosphorus retention was also conducted with broiler chicks. Day-old chicks were fed a total of 6 diets for three weeks. Phosphorus retention was measured over a four day period after feeding test diets for 7, 14 and 21 days. Diets included: (1) control (2) phosphorus deficient (3) phosphorus deficient + phytase (4) phosphorus deficient + phytase (5) phosphorus deficient + phytase + contaminated grains (6) phosphorus deficient + phytase + contaminated grains + 0.2% Mycosorb.
This study was conducted to determine any effect of trichothecene mycotoxins such as vomitoxin on the efficacy of exogenous phytase. Trichothecenes have been shown to cause lesions in the intestinal epithelium and the efficacy of phytase might, therefore, be compromised. Results are given in Table 4.
Addition of phytase to phosphorus deficient diets improved phosphorus retention but this effect was greatest when phytase was fed together with contaminated grains. The feeding of mycotoxin adsorbent reduced this effect. The reason for these results is not clear. It is possible that the contaminated grains contained live fungal spores with phytase activity.
Feeding trials with starter pigs
In the summer of 2000, an experiment was conducted at the University of Guelph Arkell Swine Research Centre to determine the potential for Mycosorb to overcome the toxicity of diets containing blends of grains naturally-contaminated with deoxynivalenol and fusaric acid.
Purebred Yorkshire pigs (average initial weight 8.1 kg) were fed diets formulated to contain 3.0 mg deoxynivalenol / kg and 20.0 mg fusaric acid / kg for 21 days. Diets included: (1) control (2) contaminated grains and (3) contaminated grains + 0.2% Mycosorb.
There was a highly significant reduction in weight gain and feed intake of pigs fed contaminated grains compared to controls (Table 5). These differences were too large large, however, to be prevented by the feeding of 0.2% Mycosorb. Higher levels of the product might have been more effective. The feeding of Mycosorb did prevent the significant depression in liver weight seen with the feeding of contaminated grains.
At the end of the study, a subgroup of 12 pigs fed each diet was euthanized and brains were excised and dissected into frontal cortex, pons and hypothalamus. Brain regional neurochemistry was determined by high performance liquid chromatography with electrochemical detection. Effects of diet were seen in the hypothalamus and pons (Table 6).
The feeding of contaminated grains reduced pons norepinephine concentrations. This is likely due to the inhibitory effect of fusaric acid on the activity of dopamine-betahydroxylase, the enzyme that catalyzes the synthesis of norepinephrine from dopamine.
Also seen was an increase in the ratio of 5-hydroxyindoleacetic acid to serotonin (HIAA/5HT). This ratio can be used, with caution, as an index of serotonergic neuronal firing and related behaviors such as loss of appetite, lethargy and loss of muscle coordination.
In the hypothalamus, the HIAA/5HT ratio was also increased by the feeding of contaminated grains while concentrations of dopamine were reduced. The feeding of 0.2% Mycosorb prevented all of the neurochemical changes seen when contaminated grains were fed.
| Conclusions The active component of choice in commercial preparations for overcoming mycotoxin contamination of feeds is a mycotoxin binding agent. In the absence of an effective binding agent, the feeding of grains contaminated with Fusarium mycotoxins results in harmful metabolic changes that reduce production efficiency of broilers, swine and other species. Our studies have shown that Mycosorb effectively prevents such metabolic changes by preventing intestinal absorption of a mixture of mycotoxins of widely varying molecular weights and charges. |
| Table 1. Fusaric acid content of cereal grains and whole feedsa. |
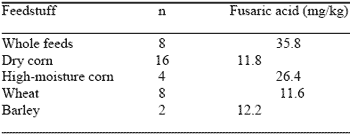 |
| aFrom Smith and Sousadias, 1993. J. Agr. Food Chem 41:2296. |
| Table 2.Effect of feeding blends of Fusarium mycotoxin-contaminated grains on growth performance of broiler chickens. |
 |
| bDeoxynivalenol. cFusaric acid. dg/kg. eOrganic polymer. fgMeans within a column without a common superscript differ significantly (P<0.05). |
| Table 3. Effect of feeding blends of Fusarium mycotoxin contaminated grains on blood metabolites and breast meat coloration of broiler chickens. |
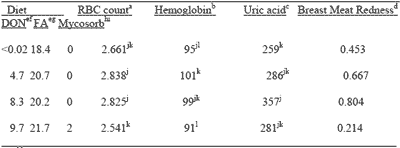 |
| a1012/L. bppm. cumol/L. dScale of 0 to 1 (green to red). emg/kg. fDeoxynivalenol. gFusaric acid. hg/kg. iOrganic polymer. jklMeans within a column without a common superscript differ significantly (P<0.05). |
| Table 4.Phosphorus retention (%) in broilers fed diets containing grain contaminated with Fusarium mycotoxins. |
 |
| Table 5. Effect of feeding blends of Fusarium mycotoxin-contaminated grains on growth performance and liver weights of starter pigs. |
 |
| akg per pig in the first 7 days. bkg per pen in the first 7 days. cdMeans within a column without a common superscript differ significantly (P<0.05). eDeoxynivalenol. |
| Table 6.Effect of feeding blends of Fusarium mycotoxin-contaminated grains on brain neurochemistry of starter pigs. |
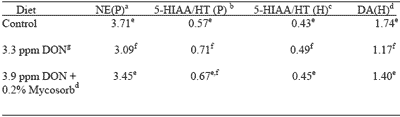 |
| aNorepinephrine (nmol/g pons tissue). bThe ratio of 5-hydroxyindoleacetic acid to serotonin in pons tissue. cThe ratio of 5-hydroxyindoleacetic acid to serotonin in hypothalamus tissue. dDopamine (nmol/g hypothalamus tissue). e,fThe means within a column without a common superscript differ significantly (P<0.05). gDeoxynivalenol. |
References
Bacon, C.W., J.K. Porter, W.P. Norred, and J.F. Leslie. 1996. Production of fusaric acid by Fusarium species. Appl. Environ. Microbiol. 62:4039.
Carson, M.S., and T.K. Smith. 1983a. Role of bentonite in the prevention of T-2 toxicosis in rats. J. Anim. Sci. 57:1498.
Carson, M.S., and T.K. Smith. 1983b. Effect of feeding alfalfa and refined plant fibres on the toxicity and metabolism of T-2 toxin in rats. J. Nutr. 113:304.
James, L.J., and T.K. Smith. 1982. Effect of dietary alfalfa on zearalenone toxicity and metabolism in rats and swine. J. Anim. Sci. 55:110.
Matsui, Y., and M. Watanabe. 1988. Quantitative analysis of fusaric acid in the cultural filtrate and soybean plants innoculated with Fusarium oxysporum var. redolens. J. Rakuno Gakuen Univ. Nat. Sci. 13:159.
Smith, T.K. 1984. Spent canola oil bleaching clays: potential for treatment of T-2 toxicosis in rats and short-term inclusion in diets for immature swine. Can. J. Anim. Sci. 64:725.
Smith, T.K., E.G. McMillan, and J.B. Castillo. 1997. Effect of feeding blends of Fusarium
mycotoxin-contaminated grains containing deoxynivalenol and fusaric acid on growth and feed consumption of immature swine. J. Anim. Sci. 75:2184.
Smith, T.K., and M.G. Sousadias. 1993. Fusaric acid content of swine feedstuffs. J. Agr. Food Chem. 41:2296.
Stangroom, K.E., and T.K. Smith. 1984. Effect of whole and fractionated dietary alfalfa meal on zearalenone toxicosis in rats and swine. Can. J. Physiol. Pharmacol. 62:1219.
Wood, G.E. 1992. Mycotoxins in foods and feeds in the United States. J. Anim. Sci. 70:3941.
Wu, W., D. Jerome, and R. Nagaraj. 1994. Increased redness in turkey breast meat induced by Fusarial culture materials. Poultry Sci. 73:331.
Authors:Trevor K. Smith1, Ewen J. MacDonald2, Swamy Haladi1 and Maher Zaytoun1
1 Department of Animal and Poultry Science, University of Guelph, Ontario, Canada
2 Department of Pharmacology and Toxicology, University of Kuopio, Kuopio, Finland







