I. INTRODUCTION
Mycotoxin-contaminated feeds are frequently reported to be more toxic for animals than their purified forms, indicating possible interactions with each other or with the micro-organisms in the animal’s gastrointestinal tract (JECFA, 2002) as bacteria (Galarza-Seeber et al, 2016). The gastrointestinal tract is the largest immune organ, where several immuno-regulatory mechanisms simultaneously defend the body (Belkaid & Hand, 2014). Yet, it is also home to a diverse community of bacteria, fungi, protozoa, and viruses. GNB are part of the microbiota, thus LPS ‘the major constituent of the outer membrane of all GNB’ are present in the intestine as GNB continuously multiply and die (Van Amersfoort, 2003). Under eubiosis, LPS does not affect animals because intestinal epithelial cells are poorly responsive to LPS when stimulated from the luminal side. However, when LPS crosses the intestinal barrier, reaches the bloodstream or emerges in the basolateral (BS) membrane, inflammatory cytokines are upregulated and stimulate the expression of toll-like receptor (TLR)-4 in the apical (AP) side of the intestinal cells. The immune response starts and changes in the epithelial structure and functionality occur (Ghareeb et al., 2016; Vamadevan et al., 2010; Zhang et al., 2017).
LPS in the gut can enter the circulation mostly by paracellular transport in the intestinal epithelium ‘enhanced by a dysfunctional barrier’ but also by transcellular transport without a barrier disruption (Mani, 2012). Once LPS is in the bloodstream, it induces inflammation and fever, sometimes followed by shock and, eventually,septicemia and death. These inflammatory responses require increased cytokine levels, whose activation is mediated by TLR-4, the immune receptors that recognize LPS (Sampath, 2018) and culminates with phosporylation and activation of the transcription factor NF-kB. A high portion of energy is utilized to sustain the elevated immune response (Li et al., 2015); thus, LPS depress growth performance and feed efficiency. LPS also triggers or exacerbates diverse diseases or disorders that hinder animal health, such as heat stress and oxidative stress (Huang, 2017) and they mediate immune-pathological alterations in the liver, the bursa, and the intestine, as well as in the reproductive tract.
Compounds known as mycotoxin binders can adsorb small molecules during passage through the digestive tract, resulting in the excretion of a toxin-adsorbent complex in the faeces (Kolosova and Stroka, 2011). A mixture of clay, yeast cell walls and phytomolecules has recently been shown to have efficacy in reducing the effects of feed-borne mycotoxins in poultry (Janječić et al., 2020). Likewise, some compounds have been tested for their ability to mitigate the adverse effects of LPS (Basauri et al, 2019; Farkas et al., 2014), such as sodium bentonite, palygorskite and organoclays, which have also been tested for their ability to bind mycotoxins (Chen et al, 2020; Schaumberger et al, 2014; Zheng et al, 2020). These results indicate the opportunity to use specific toxin binder products as a practical and economic measure to reduce LPS challenges under conditions of stress and/or mycotoxin exposure in animals including poultry.
The objectives of the current study were to evaluate the effect of LPS in an in vitro model of IPEC-J2 cells and the possible mitigating and anti-inflammatory activity of an anti-mycotoxin product in the same model.
II. METHOD
Intestinal porcine epithelial-jejunum (IPEC-J2) cells were seeded and differentiated on semipermeable Transwell filters of 12 mm diameter, 0.45 μm pore diameter, fitting in 12-well plates. This filter system allows the separation of the AP from BL compartments. Four different solutions were prepared using serum-free cell culture medium, the IPEC-J2 growth factor solution (GFS): Control (C): only growth factor solution (GFS); GFS + an anti-mycotoxin product (Mastersorb Gold, from EW Nutrition GMBH (MS)); LPS: GFS, 20µg/ml lipopolysaccharide (LPS; serotype O111:B4 from E. coli), and LPS+MS: GFS, 20µg/ml lipopolysaccharide (LPS; serotype O111:B4 from E. coli), 1mg/ml MS (≙0.1%).
All solutions were incubated for 2.5 h at 37°C with shaking. After centrifugation, the supernatants were added to the AP side of the filters containing the IPEC-J2, and incubated for 4 and 24 hours at 37°C. The solutions of all samples were collected, NF-kB activation after 4 hours was evaluated by immunofluorescence and Interleukin-8 (IL-8) after 24 hours was quantified by ELISA.
Cell monolayers were stained with fluorescent specific antibodies targeting occludin (as marker of tight junction integrity) and P-p65 (indicating the activation -phosphorylation- of NF-kB). To localize the proteins, rabbit polyclonal anti-P-p65 antibody and mouse monoclonal anti-occludin antibody were used. For secondary detection, Tetramethylrhodamine (TRITC) was used for P-p65, and Fluorescein-5-isohiocyanate (FITC) for occludin. Nuclei were stained with 4', 6-Diamidino-2- Phenylindole (DAPI). Stained monolayers were mounted on glass slides and analyzed under a confocal microscope.
All experiments were performed in triplicate. Prior to analysis, normal distribution and homogeneity of variance of all variables were assumed with Shapiro-Wilk’s and Levene’s tests. Statistical significance followed by post hoc Tukey honestly significant difference (HSD) test.
III. RESULTS
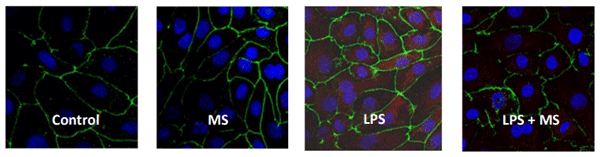
The immunofluorescence experiments showed nuclear localization of phospho-NF-κB p65 (Pp65) upon LPS stimulation, that was not present in cells simultaneously treated with LPS and clay (Figure 1). The P-p65 translocation did not occur after the addition of the clay alone to the cells.
Figure 1 - Immunofluorescence of NF-kB (P-p65) in IPEC-J2 cells treated for 4 h either with and without an LPS challenge and with and without an anti-mycotoxin product. Red: p-p65-TRITC (Tetramethylrhodamine). Green: occludin-FITC (Fluorescein-5-isothiocyanate). Blue: nucleiDAPI (4', 6-Diamidino-2- Phenylindole).
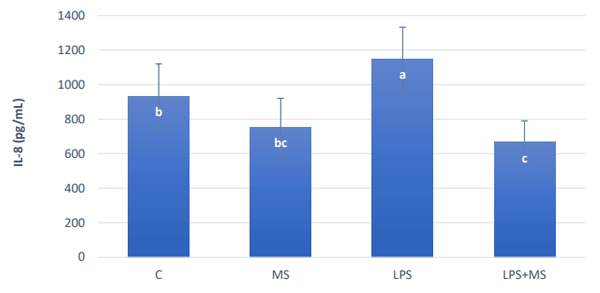
The challenge of 20µg/ml E. coli LPS (serotype O111:B4) increased significantly (P < 0.05) the production of IL-8 24 hours after the challenge (Figure 2). MS alone did not increase inflammation and lower amounts of IL-8 were detected in the solution collected from the IPECJ2 cells exposed to the LPS together with MS.
Figure 2 - IL-8 secretion of IPEC-8 cells 24 after an exposure to different solutions with and without an LPS challenge and with and without an anti-mycotoxin product. Different letters indicate significant differences (p≤0.05).
IV. DISCUSSION
IPEC-J2 cells have been used as an intestinal in vitro cell model showing inflammatory reactions (Xu et al., 2020). The increased expression and circulation of pro-inflammatory cytokines triggered by LPS is detrimental for the animal (Rauber et al., 2014). The results of the experiment, in regards with induction of inflammatory response, confirmed those of Palocz et al. (2016) who found significant increase of IL-8 in IPEC-J2 cells 24 hours after LPS treatment (10 μg/ml). In the present experiment, an increase of 20% in IL-8 release was found with a higher dose of LPS, but lower doses also increased IL-8 as shown by Geens and Niewold (2010). Moreover, using a chicken ileal explant culture model, Zhang and his team (2017) observed that a challenge of 20µg/ml induces an acute inflammatory response after 2 hours of incubation, including an increase of 80% in IL-8.
Binding LPS in the gut lumen may reduce the load of endotoxins and the risk of them entering the bloodstream (Ditter et al, 1983). An evaluation of binding substances in in vitro conditions provides a screening point and higher insights into the mode of action (Schaumberger et al, 2014). The binding of LPS by commercial products is so far, not widely explored; a few traditional binding experiments were performed in artificial intestinal fluid quantifying endotoxins with the Limulus Amebocyte lysate (LAL) test (Schaumberger et al, 2014).
The use of the IPEC-J2 cell model, in this experiment, confirmed that MS is innocuous to the cell-line and demonstrated the beneficial effect that MS potentially has at intestinal level in binding the LPS and reducing inflammation, evidenced by the lower immunofluorescence of NF-kB and IL-8 levels in the MS and in the MS+LPS groups, respectively.
Reducing inflammation factors, such as LPS, in production animals, increases health and welfare and helps to ensure that nutrients are being used for growth and muscle development instead of fighting the challenge.
Further experiments with avian intestinal cell lines should be performed to confirm the application of the results of the study in poultry. However, the use of healthy intestinal cell line-models such as IPEC-J2, to study the mechanisms of existing harmful substances and their mitigation strategies, is an advantageous approach. The findings can be extrapolated to other species (Palocz et al., 2016), experimental costs are reduced and ethical considerations of animal welfare are moot, as no animals are required.
Presented at the 32th Annual Australian Poultry Science Symposium 2021. For information on the next edition, click here.