INTRODUCTION
Fungi, especially filamentous fungi can engender secondary metabolites denominated mycotoxins that have deleterious impacts, such as estrogenic effect, carcinogenicity, teratogenicity and mutagenicity in humanity and animal. Secondary metabolites of filamentous fungi can be more or less artificially divided in antibiotics that are toxic for microorganisms, phytotoxins that are toxic for plants along with mycotoxins that are toxic for human and animal. Environmental conditions are the major factors affected mycotoxins excretion in field area, post-harvest area, storages, processing and through transportation. For example, sharply rising in temperature and relative humidity will reflected as more enhanced in toxigenic fungi growth and toxins production in comparing to the effect of those factors in bacterial case. Molds can be found in agriculture soil and when the seed kernel is damaged, the fungus can invade and multiply. Many environmental stresses on the plants during the cultivation such as drought conditions motivate insect damages and/or toxigenic fungi growth.
Mycotoxigenic fungi grow better under certain environmental conditions. However, the presence of these fungi does not mean the mycotoxins synthesis, as creation of secondary metabolites is not substantial to the synthesizing organism. Actuality, the conditions in which these molds excrete their mycotoxins are very concrete and independent of those required for fungal magnification. Conversely, the obvious absence of mold does not denote that no mycotoxins are present since these may remain in the product long after arise fungus has vanished. It is not possible to entirely avert the formation of mycotoxins, but eliminating the conditions obligatory for fungal magnification avails obviates formation of the toxin.
Ochratoxin A (OTA) was first described as a toxic metabolite excreted by Aspergillus ochraceus, awarding this secondary metabolite its name. The OTA is a secondary metabolite of some toxigenic storage fungal species of the genera Aspergillus and Penicillium. It has a prevalent occurrence in foods and feedstuffs. Particularly in recent years, ochratoxin A had more care, thus lead up to improve the knowledge for this mycotoxins kind. According to Pfohl-Leszkowicz and Manderville, ochratoxin is fundamentally considered as a nephrotoxic toxin along with this, it has hepatotoxic, teratogenic and immunotoxic actions. Petzinger and Weidenbach recorded that, according to mycotoxins hazard classification, ochratoxin A was deemed to be class 2B human carcinogen grade. Also, ochratoxin A was considered as an important cause of some endemic diseases, some area like Balkan had infected by those endemic diseases, it was called Balkan endemic nephropathy, this kind of disease had symptoms seemed to be more closely to ochratoxin infected symptoms. Pfohl-Leszkowicz et al., appearing of ochratoxin on Egyptian wheat grain in this situation will considered as a factor for increase the infection by this disease.
The presence of OTA has been reported in a number of plant products and occasionally in body fluids and kidneys of animals and humans. It has been commonly found in cereals but it can also contaminate a variety of other plant and animal products. Wheat and barely contain important amounts of OTA, in particular rye. The OTA has been shown to occur in various grains and other plant products throughout the world. It is generally spotted that concentrates are more vulnerable to the growth of fungi that able to producing OTA, especially in cereal feeds such as corn, oats, barley or wheat and in mixed feed. The OTA seems to happen more frequently and with a tendency for higher contents in mixed feeds compared to cereal grains, although it originates dominantly from grains in this feed group.
Magan and Lacey realized that, environmental factors such as wind, temperature, pH value, nutritional factors and other ones, also affect the growth of mycelia and the toxin producing by fungi. Temperature and water activity (aw) are the main factors affecting germination, growth and sporulation of toxigenic fungi. Growth of ochratoxin kinds of fungi such as A. ochraceus and OTA formation has been shown to be influenced by temperature, aw and other agents in different food and feed materials such as cereals. The impact of climate change on fungal colonization has not been yet specifically and thoroughly addressed, temperature, humidity and precipitation are known to have an effect on toxigenic moulds and on their interaction with the plant hosts.
Generally it is well known that changes in temperature ranges in any region is directly reflected on growth and metabolites of mycotoxigenic fungi activities, as well as each toxigenic fungi had optimum temperature which had better perform within it, carry out fungi to produce toxins is related with temperature change within regions. Frequent of hot and dry seasons in Italy have performed to increasing appearance of Aspergillus flavus, which considered as common Aspergillus species xerophilic member with consequent unlooked for aflatoxin toxicity sharp outbreak, thus was strange in Europe, especially in the Southern areas. Also in United States, severe outbreaks of aflatoxins have been found for the same reasons. Generally moist, humid conditions favor mould growth, moist conditions following periods of heavy precipitation or floods would be expected to favor mould growth. Generally speaking, conditions adverse to the plant (drought stress, stress induced by pest attack, poor nutrient status, etc.) encourages the fungal partner to develop more than under favorable plant conditions with the expectation of greater production of mycotoxins. This study was aimed to explain how the changes in climate condition reflecting on food safety and security, also changes in climate reflecting on changes in toxigenic fungi and mycotoxins excretion, this will help to mitigate the risk of mycotoxins.
MATERIALS AND METHODS
Basic apparatus, chemicals and solvents: Conical flask, measuring cylinders, vials, glass funnel, buchner funnel, screw cap lined with teflon, shaker, model EL680, Eberbach Co., rotary evaporator system Cole-Parmer, diagonal, 115 VAC, high-speed blender (15000 rpm) with a 1 L glass jar and cover (General electric), a model JFSD, 100 grinding/sub-sampling mill for wheat seed (EW-28615-00), UV light chamber, micropipette (5-100 µL adjustable). Cleanup cartridges were presented from Romer labs., Inc. (USA). The HPLC grade water was prepared with a ZD20 four bowl Milli-Q water system (Millipore), chromatography column, 25 mm (i.d.)×300 mm length.
Isolates fungi: All the fungi used in this study were isolated from Egyptian wheat during the years 2009-2014. Potato Dextrose Agar (PDA) supplemented with 60 µg mL-1 chloramphenicol (PDAC) was used for isolation of fungi. Ten plates of PDAC for each sample were embedded by 10 seeds on each plate.
Isolates were classified as described by Domsch et al. and Samson. When the fungus is identified to genus level, different identification procedures may be used. Penicillium and Aspergillus identification are usually inoculated on the media Czapek yeast extract agar (CYA), Malt Extract Agar (MEA), yeast extract sucrose agar (YES). The cultural characteristics of the selected fungal isolates on PDA media were recorded. The fungal isolates were also examined microscopically. The slide agar method according to Benson was used for preparation of isolates. Slides were examined microscopically under low and high power after staining with lactophenol cotton blue stain.
Inoculation and incubation:Before plating, hold sample at -20ºC for 72 h to kill mites and insects that might interfere with analysis, then sieve were used to getting rid of it. Isolates were grown on Czapek yeast extract agar (CYA) medium at 25ºC for 5 days. Conidia were collected by scraping in an aqueous 0.005% tween 80 solution to 106 spores mL-1 , determined by a haemocytometer slide and used for inoculation dichloran 18% glycerol (DG18) agar plates (15 mL, 6 cm in diameter). From each sample, transfer about 50 g into a sterile 300 mL beaker. Using 95% ethanol-flamed forceps place intact food items on surface of solidified agar, 5-10 items per plate. Flame the forceps between plating of each item. Antibiotic was added to media to inhibit bacterial growth that may interact with fungi. Chloramphenicol is the antibiotic of choice, because it is stable under autoclave conditions. Therefore, media preparation is easier and faster due to the elimination of the filtration step. The recommended concentration of this antibiotic is 100 mg L-1 medium.
Toxin production:Into 300 mL wide-mouth Erlenmeyer flask, add 50 g homogenized milled wheat and 50 mL distilled water. Plug flasks with cotton and autoclave 20 min at 121ºC and 15 psi. Aseptically multispore-inoculation separate cooled flasks with individual mold isolates. Incubate inoculated flasks at 22-25ºC until entire surface is covered with growth and mycelium has penetrated to bottom of flask (15-20 days).
To each flask, add 150 mL chloroform, using short-stem glass funnel inserted alongside unremoved cotton plug (to minimize mold spore dissemination). Heat flask contents in fume hood on steam bath until solvent begins to boil. With spatula, break up moldy cereals cake and transfer flask contents into explosion-proof blender and blend at high speed for 1 min.
Filter blender contents through filter paper inserted into short-stem glass funnel. Collect filtrate in 300 mL Erlenmeyer flask. Return cake to blender, add 100 mL unheated solvent and blend 1 min at high speed. Filter as above and combine filtrates. Add boiling chips to flask containing filtrates and evaporate with steam to 20-25 mL. If analysis is not to follow immediately, evaporate to dryness and store flask in the dark. Rinse all glassware, etc., used for extraction in 5% NaOCl solution before soap and water cleansing. Submerge cake in 5% NaOCl solution for 72 h before autoclaving and disposal.
OTA extraction from culture:Ochratoxin A was extracted by a variation of Bragulat et al. method. After 15 and 20 days of incubation, three agar plugs (diameter 0.5 cm) were removed from the inner, middle and outer area of each colony. Plugs were weighed and introduced into 3 mL vials. Methanol (1 mL) was added and the vials were shaken for 5 sec (Autovortex SA6, Surrey, UK). After being left stationary for 60 min, the extracts were shaken again, filtered (MillexR SLHV 013NK, Millipore, Bedford, Massachusetts, USA) and injected into a HPLC instrument (Waters, Milford, Massachusetts, USA).
Detection and quantification of OTA by HPLC:The production of OTA was detected and quantified by HPLC with fluorescence detection (λexc, 330 nm; λem, 460 nm) (Waters 474), using a C18 column (Waters Spherisorb 5 µm, ODS2, 4.6×250 mm). The mobile phase (acetonitrile-water-acetic acid, 57:41:2) was pumped at 1.0 mL min-1 . The injection volume was 25 µL and the retention time was around 7 min. The detection limit of the analysis was 0.01 µg g-1 OTA. Quantification was achieved with a computing integrator (Millenium-32 v. 3.05 software, Milford, Massachusetts, USA). The OTA was quantified on the basis of the HPLC fluorometric response compared with that of a range of OTA standards.
Statistical treatment of the results: The OTA concentrations detected at each condition were evaluated by analysis of variance using M Stat program version 6.
RESULTS AND DISCUSSION
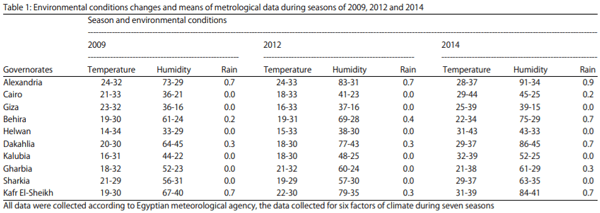
Many climate parameters were recorded to standup with probability of changes that could happen, those parameters were temperature, relative humidity, wind speed, rainfall and dew point. The average of three parameters that were temperature, humidity and rains fall were recorded as for the selection three seasons appeared in Table 1. The results showed that, the average of temperature range all over the ten governorates were changed by the seven seasons. Averages of major and minor temperatures varied from year to the other, while it was mostly in a marked increase over the last two seasons, also these ranges were not stable for the ten governorates according to its position.
In the first season of 2009, Helwan major temperature range was highest one of the ten governorates, it has also a big difference between major and minor temperature values; otherwise, the lowest governorate in temperature range values was Sharkia. These results were changed according to seasons and to the governorate position, by the last season 2014, the highest governorate in major value was Cairo and the lowest one was in Behira (Table 2, 3). Other metrological data was also changed, the relative humidity also had higher values; Alexandria and Dakahlia were the governorates with highly relative humidity values. On the other hand, Cairo and Helwan were the lowest governorates in relative humidity values (Table 1).
The rainfall had fluctuated during this period but all over the seven season it was increased, there were three governorates recorded the maximum amount of rainfall. Alexandria at precipitation value of 0.9 mL followed by Kafr El-Sheikh and Dakahlia at precipitation value of 0.7 mL and four governorates recorded the lowest precipitation value of 0.0 mL, these governorates were Sharkia, Kalubia, Helwan and Giza. The effect of those parameter changes on toxigenic fungal growth and mycotoxins production were studied and the data recorded (Table 2, 3, Fig. 1).
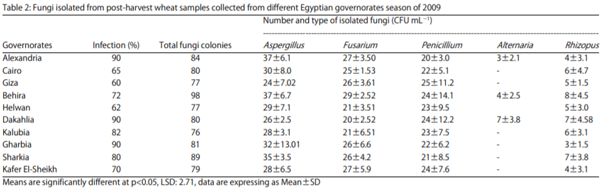
The fungal isolation were tested for wheat as a crop of cereals during the same period and the number of isolated fungi were recorded for the ten governorates, types of toxigenic fungi were varied from governorate to other, also the total fungal growth were changed from one season to the other. Total Fungal Colony (TFC) of wheat was recorded in all seasons from 2009-2014. First for wheat samples, in 2009 three governorates had high infection ratio at 90%, the highest number of total fungal count was 98 CFU mL-1 and it was found in Behira, the lowest total fungal count was 77 CFU mL-1 and it was found in two governorate Helwan and Giza (Table 2).
Aspergillus and Penicillium species were appeared as >50% of total fungal count, these geniuses included the ochratoxin producing strains. Alternaria sp. was found to be appeared in three governorates Alexandria, Behira and Dakahlia, these governorates are near costal governorates and its positions are on the North of the country. Rhizopus sp. was appeared as from 3-8 CFU mL-1 in the ten governorates. During the next seasons, there is some changes were happened in environmental conditions. These changes were in temperature, humidity and rainfalls level. The changes not so great but it has effect on the number of fungi, types and amount of toxin excreted.
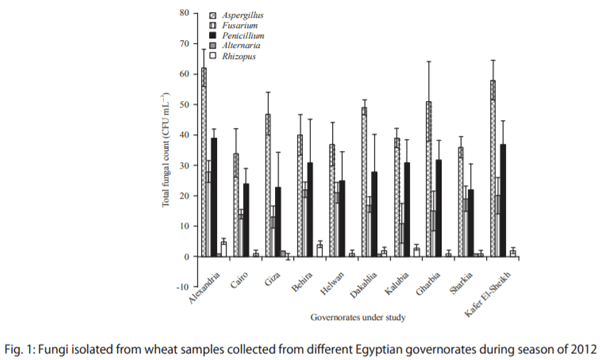
In the results of season 2012, high infection ratio of the sample was 100% and it appeared in Alexandria governorate, the highest governorate in Total Fungal Count (TFC) was in Alexandria and it was 137 CFU mL-1 , otherwise, the lowest governorate in total fungal count was Cairo, the number of TFC was 73 CFU mL-1 . It was found that, total fungal count of Aspergillus all over ten governorates was increased, also Penicillium sp. was appeared to be more infection in total fungal count of fungal isolated from samples (Fig. 1). By refereeing to the data that were collected from Egyptian General Authority of Meteorology of the year 2012, the Meteorological data in 2012 was found that: There was a change in maximum and minimum temperatures values comparing to the same period with 2009 values. Temperatures rates were heating on the Northern regions of the Egypt country, it was higher than values that recorded during the same period in 2009, while the levels of relative humidity and dew point values which recorded in 2012 were much higher than those recorded in data of 2009. Those factors of climate is already considered also as factors that affecting on fungal growth and mycotoxin production. It was sound as changes on the toxin excretion amount and it sometimes reflected on the fungal type that will appeared on the crops of this area.
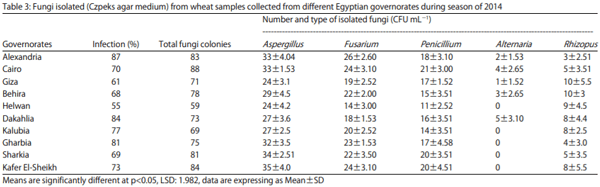
By the season of 2014, high infection ratio of the sample was 87% and it was found in Alexandria governorate, the highest governorate in total fungal count was Cairo and it was at 88 CFU mL-1 . The lowest governorate in total fungal count was Helwan and the total fungal count was 59 CFU mL-1 . It was found that, total fungal count of Penicillium all over ten governorates was decreased, also Alternaria sp. was appeared in two of the middle governorate Cairo and Giza, those governorates has a large distance from the coastal area (Table 3).
According to Abdel-Hafez et al. for the fungi presence on wheat and sorghum dust, the results recorded the presence of different types of toxigenic fungi such as Aspergillus (especially A. ochraceus), Fusarium, Penicillium and Alternaria. Also found in that study that Aspergillus was the dominant fungi on wheat dust followed by Penicillium species. The total counts of fungi in wheat dust were recorded as 35420 fungi variation as 14 genera, these genera were included 19 species and 4 varieties.
Ochratoxin A was also tested in wheat samples of the ten governorates in the first season and the results showed that, the high ratio of contamination was up to 60% on samples under inspection and it was found in Kafr El-Sheikh (Table 4). Five governorates were recorded at infection ratio of 40%, contaminated samples were determined in two samples of the five samples that collected from those governorates under test plan, governorates were Alexandria, Cairo, Helwan, Dakahlia and Sharkia, by highly positive sample in Helwan that was 14.2 µg kg-1 ochratoxin A . Other four governorates had a ratio of contamination of 20% of sample under inspection.
Higher concentration of ochratoxin A on wheat grains were found in Kafr El-Sheikh at levels of 24.9, 25.6 and 21.4 µg kg-1 of wheat sample for years 2009, 2012 and 2014, respectively. Otherwise, the lowest concentration was found in Cairo at levels of 4.55, 4.32 and 3.1 µg kg-1 of wheat sample for years 2009, 2012 and 2014, respectively. Ochratoxin A concentration all over the ten governorates were ranged between 2.1-24.9 µg kg-1 in season of 2009 otherwise in 2012 it was between 2.5-25.6 µg kg-1 but the range was decreased in 2014 to 1.3-21.4 µg kg-1 of wheat samples (Table 4).
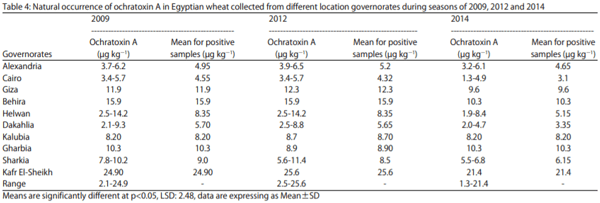
It is mentioning that El-Sayed et al. in survey on cereal grains and animal tissues from Egyptian market found that, ochratoxin A was not detected on either wheat or wheat flour. Abdelhamid, collected several kind of food samples for ochratoxin A analysis, in 51 samples it was 12 positive for ochratoxin detection by percentage ratio of 23.5%, in wheat the level of contamination was 10 µg kgG1 and in wheat bran it was 6 µg kg-1.
Speijers and van Egmond reported in a survey of ochratoxin A levels in food and feed in African countries that, in Egypt many food and feed commodities were found to be contaminated with ochratoxin A. This study will help the researcher to uncover the critical hazard of climate change on food grains, along with it uncover the critical area which suffering highly contaminated, that can be beneficial for the producer to use a mitigate action, also for the importer area to modify the inspection system according to this changes.
In comparing with some data about ochratoxin A in Egypt, Ahmed et al. studied the levels of ochratoxin A in green and roasted beans of coffee, the results recorded the ochratoxin A reached 5.66 µg kg-1 with mean at 1.55 µg kg-1 in green beans otherwise the roasted beans levels were 5-8.35 µg kg-1 by mean of 6.1 µg kg-1. The toxigenic fungi of coffee beans was also studied and it was appeared as four genera of mycotoxigenic fungi that belonged to its eight species, Aspergillus was the main dominant one by four species (A. niger, A. ochraceus, A. flavus and A. parasiticus).
CONCLUSION
In comparing to the three parameters of environmental conditions changes during the last 5 years from 2009-2014, there were changes depending on climate changes happened. The data of toxigenic fungi on wheat grain was recorded as to study the dominant fungi, also to record the changes in mycotoxins levels. Ochratoxin A is appeared as a new mycotoxins contaminant on Egyptian wheat grains, the level of ochratoxin A was varied between 2.1-24.9 µg kg-1 in season of 2009, it was decreased in range to be between 1.3-21.4 µg kg-1 on season of 2014, referring to meteorological data, the average of temperature and humidity was turned increased in 2014 comparing to 2009 where it was less. Those results lead to support the thesis that changes in climate, in other word changes in environmental conditions will reflected in more or less mycotoxins toxicity and/or toxin producing fungi contamination.
SIGNIFICANT STATEMENT
Since environmental condition deemed important factor which affected by climate changes as well as toxigenic fungi and mycotoxins production, important cereal grains contaminated by new types of fungi that are not regular found in this area. According to that, climate changes considered as a major hazard for food safety and security, this study focus on the challenge of mycotoxins-related to climate, how to mitigate it, what is the situation for Egyptian wheat contamination during last years.
ACKNOWLEDGEMENT
This study was supported as part of project entitled “Novel integrated strategies for worldwide mycotoxin reduction in the food and feed chains" (MycoRed project), funded by European Commission fund, under the group of FP7 program projects. Also, thanks for Dr. Antonio Moretti, Italian National Research Council, Bari, Italy, for facilitates, supports and provided insight and expertise that greatly assisted the research.
This article was originally published in Asian Journal of Scientific Research, 10: 178-185. DOI: 10.3923/ajsr.2017.178.185. This is an Open Access article distributed under the terms of the Creative Commons Attribution License. 












.jpg&w=3840&q=75)