Occurrence of relevant mycotoxins in food commodities consumed in Chile
Author details:
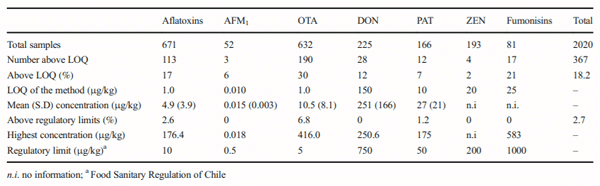
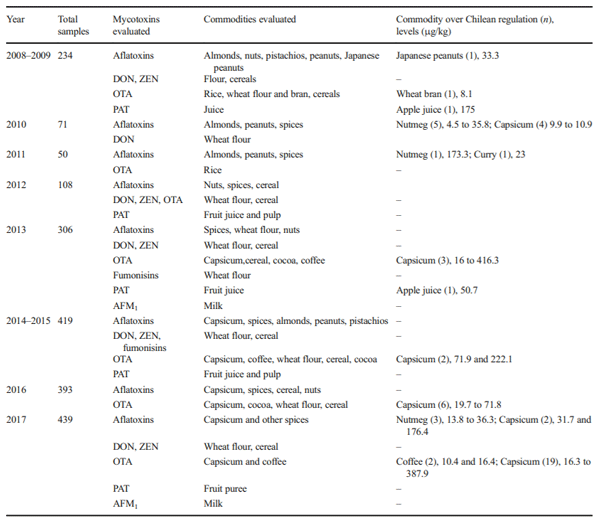
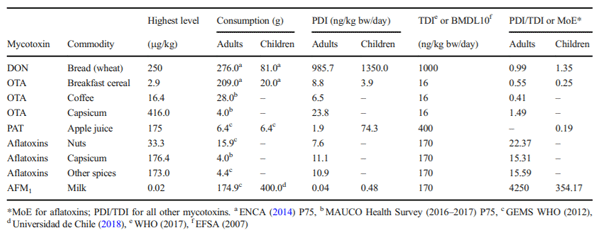
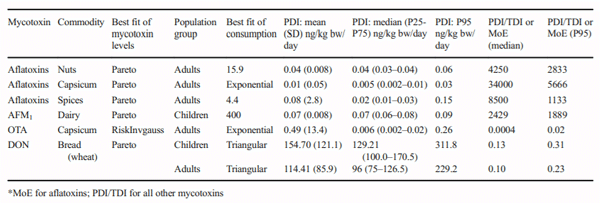
The aims of this study were to analyse the occurrence of aflatoxins, aflatoxin M1 (AFM1), fumonisins, ochratoxin A (OTA), patulin (PAT), zearalenone (ZEN) and deoxynivalenol (DON) in foodstuffs consumed in Chile between 2008 and 2017 and to estimate the contribution of main contaminated foodstuff in human exposure by the probable daily intake (PDI) estimation. In 9 years of surveillance, 2020 food samples were analysed with an occurrence of 18.2% and with 2.7% of the samples being over the Chilean regulation. The occurrence of mycotoxins in food were 16% for aflatoxins, 6% for AFM1, 30% for OTA, 12% for DON, 7% for PAT, 21% for fumonisins and 2% for ZEN. The estimated median PDI of DON because of bread consumption was 129.2 ng/kg bw/day for children and 96.0 ng/kg bw/day in adults. Median PDI because of capsicum consumption was 0.006 ng/kg bw/day for OTA and 0.005 ng/kg bw/day for aflatoxins. Median PDI of aflatoxins was estimated at 0.02 ng/kg bw/day for spices and 0.04 ng/kg bw/day for nuts consumption. In children, the median PDI of AFM1 for dairy consumption was 0.07 ng/kg bw/day. The derived margin of exposure (MoE) values ranged from 1133 to 8500 suggested that aflatoxins would be of public health concern. The PDI of the other mycotoxins did not show a health risk. This is the first survey of mycotoxins in food made in Chile; further research is needed to improve surveillance and guidelines based on national risk assessments and considering sensitive population groups.
Keywords: Mycotoxins, Occurrence, Dietary exposure, Risk assessment, Chile.
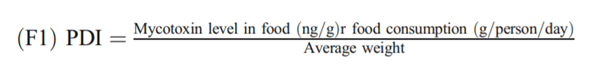
Ali N, Hashim NH, Shuib NS (2015) Natural occurrence of aflatoxins and ochratoxin A in processed spices marketed in Malaysia. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 32(4):518– 532
Asai T, Tsuchiya Y, Okano K, Piscoya A, Nishi YC, Ikoma T, Oyama T, Ikegami K, Yamamoto M (2012) Aflatoxin contamination of red chilli pepper from Bolivia and Peru, countries with high gallbladder cancer incidence rates. Asian Pac J Cancer Prev 13:5167–5170
Barreira MJ, Alvito PC, Almeida CMM (2010) Occurrence of patulin in apple-based foods in Portugal. Food Chem 121:653–658
Benford D (2016) The use of dose-response data in a margin of exposure approach to carcinogenic risk assessment for genotoxic chemicals in food. Mutagenesis 31:329–331
Bennett JW, Klich M (2003) Mycotoxins. Clin Microbiol Rev 16(3):497– 516
Berdegué F, Jara E, Modrego F, Sanclemente X, Schejtman A (2010) Comunas Rurales de Chile, Documento de Trabajo N° 60. Programa Dinámicas Territoriales Rurales. Rimisp, Santiago
Campbell HM, Armstrong JF (2007) Determination of zearalenone in cereal grains, animal feed and feed ingredients using immunoaffinity column chromatography and liquid and chromatography: interlaboratory study. J AOAC Int 90(6):1610–1622
Corcuera LA, Arbillaga L, Vettorazzi A, Azqueta A, de Cerain L (2011) Ochratoxin A reduces aflatoxin B1 induced DNA damage detected by the comet assay in Hep G2 cells. Food Chem Toxicol 49:2883– 2889
Corcuera LA, Vettorazzi A, Arbillaga L, González-Peńas E, López de Cerain A (2012) An approach to the toxicity and toxicokinetics of aflatoxin B1 and ochratoxin A after simultaneous oral administra-tion to fasted F344 rats. Food Chem Toxicol 50:3440–3446
Costa J, Rodríguez R, Garcia-Cela E, Medina A, Magan N, Lima N, Battilani P, Santos C (2019) Overview of fungi and mycotoxin con-tamination in capsicum pepper and in its derivatives. Toxins 11:27
Degen H, Partosch F, Muñoz K, Gundert-Remy U (2017) Daily uptake of mycotoxins – TDI might not be protective for nursed infants. Toxicol Lett 277:69–75
Delgado L (2011) Informe monitoreo de micotoxinas en alimentos. Subsecretaria de Salud Pública- Ministerio de Salud Instituto de Salud Pública de Chile año 2010. http://www.ispch.cl/sites/default/ files/documento/2013/05/Informe%20Micotoxinas%202010% 20vf.pdf. Accessed 17 July 2019
Delgado L (n.d.) Informe monitoreo de micotoxinas en alimentos año 2011b. Subsecretaria de Salud Pública- Ministerio de Salud Instituto de Salud Pública de Chile. http://www.ispch.cl/sites/ default/files/documento_tecnico/2012/06/informe_micotoxinas_ 2011.pdf. Accessed 17 July 2019
Delgado L, Villarroel O (n.d.) Micotoxinas en alimentos de consumo directo en Chile. Monitoreo de micotoxinas realizado por el Instituto de Salud Pública de Chile Años 2008-2009. www.ispch. cl/sites/default/files/documento/2013/05/MICOTOXINAS% 20EN%20ALIMENTOS%20DE%20CONSUMO% 20DIRECTO%20EN%20CHILE%202008-2009.pdf. Accessed 17 July 2019
EFSA - European Food Safety Authority (2007) Opinion of the scientific panel on contaminants in the food chain on a request from the com-mission related to the potential increase of consumer health risk by a possible increase of the existing maximum levels for aflatoxins in almonds, hazelnuts and pistachios and derived products. EFSA J 446:1–127 https://www.efsa.europa.eu/en/efsajournal/pub/446. Accessed 22 June 2019
EFSA - European Food Safety Authority (2010) Management of left-censored data in dietary exposure assessment of chemical sub-stances. EFSA J 8(3):1557 https://www.efsa.europa.eu/en/ efsajournal/pub/1557. Accessed 22 June 2019
EFSA - European Food Safety Authority (2013) International frame-works dealing with human risk assessment of combined exposure to multiple chemicals. EFSA J 11(7):1–69 https://www.efsa.europa. eu/en/efsajournal/pub/3313. Accessed 22 June 2019
El Darra N, Gambacorta L, Solfrizzo M (2019) Multimycotoxins occur-rence in spices and herbs commercialized in Lebanon. Food Control 95:63–70
ENCA (2014) Encuesta de Consumo Alimentario en Chile: Informe final. https://www.minsal.cl/encadescarga/. Accessed 01 December 2018 European Commission (2006) Commission Regulation (EC) no 1881/ 2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union L364:1–25 https:// e u r - l e x . e u r o p a . e u / l e g a l - c o n t e n t / E N / T X T / ? q i d = 1561986662392&uri=CELEX:32006R1881. Accessed 20 June 2019
European Commission (2007) Commission Regulation (EC) no 1126/ 2007 of 28 September 2007 amending Regulation (EC) No 1881/ 2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Off J Eur Union L 255/14. https://eur-lex.europa.eu/eli/reg/2007/1126/oj. Accessed 20 June 2019
FAO - Food and Agriculture Organization (2004) Worldwide regulations for mycotoxins in food and feed in 2003. FAO food and nutrition paper, 81. Food and Agriculture Organization, Rome. http://www. fao.org/3/y5499e/y5499e00.htm. Accessed 20 June 2019
Ferreccio C, Roa JC, Bambs C, Vives A, Corvalán AH, Cortés S, Foerster C, Acevedo J et al (2016) Study protocol for the Maule Cohort (MAUCO) of chronic diseases, Chile 2014-2024. BMC Public Health 16:122
Foerster C, Koshiol J, Guerrero AR, Kogan MJ, Ferreccio C (2016) The case for aflatoxins in the causal chain of gallbladder cancer. Med Hypotheses 86:47–52
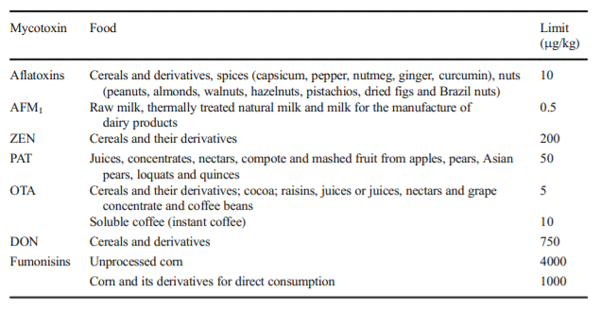
Food Sanitary Regulation of Chile (2017) Reglamento Sanitario de los Alimentos DTO. N° 977/96 (D.OF. 13.05.97) República de Chile Ministerio de Salud. http://www.dinta.cl/wp-dintacl/wp-content/ uploads/RSA-DECRETO-977-96-actualizado-25-de-mayo-2017. pdf. Accessed 20 December 2018
Franco LT, Petta T, Rottinghaus GE, Bordin K, Gomes GA, Alvito P, Assunção R, Oliveira CAF (2019) Assessment of mycotoxin expo-sure and risk characterization using occurrence data in foods and urinary biomarkers in Brazil. Food Chem Toxicol 128:21–34
Galarce-Bustos O, Alvarado M, Vega M, Aranda M (2014) Occurrence of ochratoxin A in roasted and instant coffees in the Chilean market. Food Control 46:102–107
Gambacorta L, Magistà D, Perrone G, Murgolo S, Logrieco AF, Solfrizzo M (2018) Co-occurrence of toxigenic moulds, aflatoxins, ochratoxin A, Fusarium and Alternaria mycotoxins in fresh sweet peppers (Capsicum annuum) and their processed products. World Mycotoxin J 11(1):159–174
Golli-Bennour EE, Kouidhi B, Bouslimi A, Abid-Essefi S, Hassen W, Bacha H (2010) Cytotoxicity and genotoxicity induced by aflatoxin B1, ochratoxin A, and their combination in cultured Vero cells. J Biochem Mol Toxicol 24:42–50
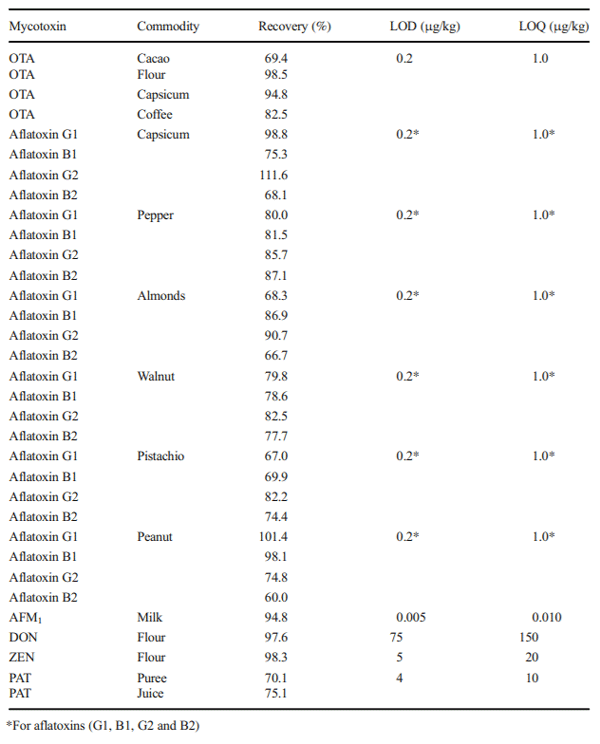
IARC- International Agency for Research on Cancer (2012) Sampling and sample preparation methods for determining concentrations of mycotoxins in foods and feeds. IARC Sci Publ 158:39–51 https:// www.ncbi.nlm.nih.gov/pubmed/23477195. Accessed 20 June 2019
Ikoma T, Tsuchiya Y, Asai T, Okano K, Endoh K, Yamamoto M, Nakamura K (2015) Ochratoxin contamination of red chili peppers from Chile, Bolivia and Peru showing high incidences of gallbladder cancer. Asian Pac J Cancer Prev 16:5897–5991
Instituto de Salud Pública de Chile (n.d.) Informe de Resultados de Vigilancia de Laboratorio Micotoxinas en Alimentos. http://www. ispch.cl/sites/default/files/documento/2013/07/Informe% 20Micotoxinas%202012%2014-06-2013.pdf. Accessed 20 June 2019
Jager AV, Tedesco MP, Souto PCMC, Oliveira CAF (2013) Assessment of aflatoxin intake in São Paulo, Brazil. Food Control 33(1):87–92
Jalili M, Jinap S (2012) Natural occurrence of aflatoxins and ochratoxin A in commercial dried chili. Food Control 24(1):160–164 Jonsyn-Ellis FE (2001) Seasonal variation in exposure frequency and concentration levels of aflatoxins and ochratoxins in urine samples of boys and girls. Mycopathologia 152(1):35–40
Karlovsky P, Suman M, Berthiller F, De Meester J, Eisenbrand G, Perrin I et al (2016) Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res 32:179–205
Kettler S, Kennedy M, McNamara C, Oberdörfer R, O'Mahony C, Schnabel J, Smith B, Sprong C, Faludi R, Tennant D (2015) Assessing and reporting uncertainties in dietary exposure analysis: mapping of uncertainties in a tiered approach. Food Chem Toxicol 82:79–95
Klarić MS, Rašić D, Peraica M (2013) Deleterious effects of mycotoxin combinations involving ochratoxin A. Toxins 5(11):1965–1987 Kumagai S, Nakajima M, Tabata S, Ishikuro E, Tanaka T, Norizuki H,
Itoh Y, Aoyama K, Fujita K, Kai S, Sato T, Saito S, Yoshiike N, Sugita-Konishi Y (2008) Aflatoxin and ochratoxin A contamination of retail foods and intake of these mycotoxins in Japan. Food Addit Contam Part A 25(9):1101–1106
Marin S, Ramos AJ, Cano-Sancho G, Sanchis V (2013) Mycotoxins: occurrence, toxicology, and exposure assessment. Food Chem Toxicol 60:218–237
Muñoz K, Vega M, Rios G, Muñoz S, Madariaga R (2006) Preliminary study of Ochratoxin A in human plasma in agricultural zones of Chile and its relation to food consumption. Food Chem Toxicol 44(11):1884–1889
Muñoz K, Campos V, Blaszkewic M, Vega M, Alvarez A, Neira J, Degen GH (2010) Exposure of neonates to ochratoxin A: first biomonitoring results in human milk (colostrum) from Chile. Mycotoxin Res 26:59–67
Muñoz K, Blaszkewicz M, Campos V, Vega M, Degen G (2014) Exposure of infants to ochratoxin A with breast milk. Arch Toxicol 88:837–846
Muñoz K, Cramer B, Dopstadt J, Humpf HU, Degen GH (2017) Evidence of ochratoxin A conjugates in urine samples from infants and adults. Mycotoxin Res 33(1):39–47
National Health Survey of Chile (2017). Encuesta Nacional de Salud http://epi.minsal.cl/encuesta-ens/. Accessed 20 December 2018 Nogueira L, Foerster C, Groopman J, Egner P, Koshiol J, Ferreccio C (2015) Association of aflatoxin with gallbladder cancer in Chile. JAMA 313:2075–2077
Ozbey F, Kabak B (2012) Natural co-occurrence of aflatoxins and och-ratoxin A in spices. Food Control 28:354–361
Prelle A, Spadaro D, Garibaldi A, Lodovica Gullino M (2014) Co-occurrence of aflatoxins and ochratoxin A in spices commercialized in Italy. Food Control 39(1):192–197
Raiola A, Tenore GC, Manyes L, Meca G, Ritieni A (2015) Risk analysis of main mycotoxins occurring in food for children: an overview. Food Chem Toxicol 84:169–180
Renwick AG, Walker R (1993) An analysis of the risk of exceeding the acceptable or tolerable daily intake. Regul Toxicol Pharmacol 18(3): 463–480
Sedova I, Kiseleva M, Tutelyan V (2018) Mycotoxins in tea: occurrence, methods of determination and risk evaluation. Toxins 10(11):444
Serra I, Yamamoto M, Calvo A, Cavada G, Báez S, Endoh K, Watanabe H, Tajima K (2002) Association of chili pepper consumption, low socioeconomic status and longstanding gallstones with gallbladder cancer in a Chilean population. Int J Cancer 102:407–411
Sherif SO, Salama EE, Abdel-Wahhab MA (2009) Mycotoxins and child health: the need for health risk assessment. Int J Hyg Environ Health 212(4):347–368
Sugita-Konsihi Y, Tanaka T, Tabata S, Nakajima M, Nouno M, Nakaie Y, Chonan T, Aoyagi M, Kibune N, Mizuno K, Ishikuro E, Kanamaru N, Minamisawa M, Aita N, Kushiro M, Tanaka K, Takatori K (2006) Validation of an HPLC analytical method coupled to a mul-tifunctional clean-up column for the determination of deoxynivalenol. Mycopathologia 161:239–243
Tsuchiya Y, Terao M, Okano K, Nakamura K, Oyama M, Ikegami K et al (2011) Mutagenicity and mutagens of the red chili pepper as gall-bladder cancer risk factor in Chilean women. Asian Pac J Cancer Prev 12(2):471–476
Universidad de Chile (2018) Preliminary study on dairy consumption in Chilean children. http://municipalidadestacioncentral.cl/wp-content/ uploads/2018/09/Estudio-de-H%C3%A1bitos-de-Consumo-de-L% C3%A1cteos-en-Ni%C3%B1os-y-Ni%C3%B1as.pdf. Accessed 7 May 2019
van Egmond HP, Jonker MA (2004) Worldwide regulations on aflatoxins—the situation in 2002. J Toxicol: Toxin Rev 23(2–3): 273–293
WHO (2002). Evaluation of certain mycotoxins in food. WHO technical report series, 906
WHO - World Health Organization (2006) World Health Organization Child Growth Standards: Methods and development https://www. who.int/childgrowth/standards/technical_report/en/. Accessed 25 February 2019
WHO - World Health Organization (2012) Global Environment Monitoring System—Food Contamination Monitoring and Assessment Programme (GEMS/Food) https://www.who.int/ nutrition/landscape_analysis/nlis_gem_food/en/. Accessed 30 May 2019
WHO - World Health Organization (2017) Evaluations of the Joint FAO/ WHO Expert Committee on Food Additives (JECFA) http://apps. who.int/food-additives-contaminants-jecfa-database/search.aspx? fcc=2. Accessed 30 May 2019
Yogendrarajah P, Jacxsens L, Lachat C, Walpita CN, Kolsteren P, De Saeger S, De Meulenaer B (2014) Public health risk associated with the co-occurrence of mycotoxins in spices consumed in Sri Lanka. Food Chem Toxicol 74:240–248
Zafar Iqbal S, Rafique Asi M, Zuber M, Akhtar J, Jawwad Saif M (2013) Natural occurrence of aflatoxins and ochratoxin A in commercial chilli and chilli sauce samples. Food Control 30(2):621–625









.jpg&w=3840&q=75)