Occurrence of Mycotoxins in Commodities collected in Spain

Mycotoxins are secondary metabolites produced by fungi on agricultural products before or after harvest. These toxic substances are known to be either carcinogenic (e.g. aflatoxin B1, ochratoxin A, fumonisin B1 (FUM)), estrogenic (zearalenone), neurotoxic (fumonisin B1), nephrotoxic (ochratoxin A), dermatotoxic (trichothecenes such as deoxynivalenol (DON) or T-2 toxin) or immunosuppressive (aflatoxin B1, ochratoxin A, deoxynivalenol, and T-2 toxin).
Mycotoxin contamination of crops may cause economic losses at all levels of food and feed production, including crop and animal production. Fusarium mycotoxin contaminated diets can cause feed refusal in farm animals, poor feed conversion, diminished body weight gain, immunosuppression, interference with reproductive capacities and residues in animal food products (Visconti 1998).
Although the prevention of mycotoxin contamination in the field is the main goal of agricultural and food industries, under certain environmental conditions the contamination of various commodities with Fusarium fungi and mycotoxins is unavoidable. Although scientific literature offers a broad variety of information on the effects of individual mycotoxins in various animal species, it is the multiple mycotoxin contamination that matters life-stock industry most, as it refers to the naturally occurring circumstances.
For example, aflatoxin and fumonisin B1, as well as DON or other trichothecenes (one or even more of them) and zearalenone frequently occur together in the same grain (Cirillo et al. 2003; Sanchis et al.; Gonzalez et al.; Norhayati et al.; Pittet, 1998). Additionally, in the feed manufacturing process different batches of different raw materials are mixed together thus producing a totally new matrix with a new risk profile in the due course of manufacturing. Poor livestock performance and/or disease symptoms observed in commercial operations may be due to the synergistic interactions between multiple mycotoxins. Scientific reports on synergistic effects of mycotoxins at acute toxicity levels describe combinations of aflatoxins with various trichothecenes, as well as with ochratoxins and fumonisins, but also combinations of fumonisins plus DON. Nevertheless it has to be pointed out, that far more work has to be done in this particular field of research, especially in the sub acute contamination range as well as with combinations of more than two toxins.
Due to new analytical methods and a growing interest in this research sector, actually more than 300 different mycotoxins are denominated. Nevertheless only a small number of mycotoxins are practically relevant, with aflatoxins, zearalenone, trichothecenes, fumonisins and ochratoxins being of particular interest (CAST, 2003; Erber and Binder, 2004).
The described survey was initiated and backed by BIOMIN® GmbH to provide customers insights in the occurrence of mycotoxins in their feed samples thereby enabling better feed management. All tests have been conducted by Quantas Analytics - Austria, a company specialized in mycotoxin analysis. Aim of this program was to identify the contamination level of certain Fusarium toxins and the co-occurrence of different types of mycotoxins which are produced by different Fusarium species. Fusarium species occurring on cereals in the field are able to produce many types of mycotoxins. The main groups of Fusarium toxins commonly recognized in grains are trichothecenes, zearalenone, and fumonisins, in which deoxynivalenol (main representative of trichothecenes) and zearalenone are mainly produced by F. culmorum and F. graminearum whereas fumonisins are mainly produced by F. moniliforme and F. proliferatum (Bottalico, 1998).
Materials and Methods
All samples were analyzed by Quantas® Analytics, a subsidiary company of Romer Labs Diagnostic GmbH, specialized in food and feed analysis. DON, ZON and total FUM were analyzed by Enzyme-Linked Immunosorbent Assay (ELISA). For the purpose of data analysis, non-detect levels are based on the quantification limits of the test method for each toxin: DON <250 µg/kg; ZON <40 µg/kg; FUM <250 µg/kg.
Samples
All analyzed samples were part of a project initiated by the feed additive producing company Biomin® GmbH in cooperation with the Spanish partner company Qualivet. Aim of the project was to survey Spanish raw materials and feed samples for occurrence and level of mycotoxins, which create major problems in animal production. Thus Spanish feed and animal producers where invited to collect feed samples of major commodities to analyze them for major mycotoxins of interest in animal husbandry, in order to provide them insights into the occurrence of mycotoxins in their feed. Samples of raw materials were taken directly at animal farms or animal feed producing sites, following the principles of the Romer® guide on “Sampling and sample preparation for mycotoxin analysis” (Richards, 2000). Even though sample providers were strongly encouraged to follow the guides, the actual sampling procedure could not be surveyed.
The results on this report were based on 90 samples collected over a 3 months period, from the end of September until November 2006. The samples received were mainly from Spain and the major Spanish import markets (France, Germany, America), and they were tested on– zearalenone (ZON), deoxynivalenol (DON), fumonisin (FUM). Data were analyzed from two perspectives: first by geographical regions and second by means of commodity types.
In order to get a better overview about toxin occurrence in different regions, data were grouped as follows: central-north Spain (area around Madrid), north-east Spain (regions of Aragòn and Cataluña). Samples were collected by Qualivet’s customers and sent for further analysis to the Quantas® laboratories in Tulln, Austria.
Instruments and Reagents
Sample extraction was done by shaking samples on a GFL shaker model 3017 purchased from GFL, Burgwedel, Germany. Photometric measurements were performed by use of a Stat Fax Reader model 303 Plus purchased from Romer Labs Diagnostic GmbH, Tulln, Austria.
For extraction of the samples distilled water was used, methanol (normapur, purchased from VWR Prolabo) and Sodiumchloride (98%, from VWR Problabo).
Sample preparation
Samples were ground using a Romer Series II® Mill (purchased from Romer Labs Diagnostic GmbH, Tulln, Austria) so that 75% passed thru a 20-mesh screen. After mixing the samples thoroughly, subsample portions of 20g were weighed into glass chars by use of a Sartorius LC 620P scale (purchased from Sartorius, Vienna, Austria).
Enzyme-Linked Immunosorbent Assay – (ELISA)
The AgraQuant® Assay is a direct competitive enzyme-linked immunosorbent assay (ELISA). Mycotoxins are extracted from a ground sample with a specific solvent. The extracted sample and enzyme-conjugated mycotoxin are mixed and added to the antibody-coated microwell. Mycotoxins in samples and control standards are allowed to compete with enzyme-conjugated mycotoxins for the antibody binding sites. After a washing step, an enzyme substrate is added and blue color develops. The intensity of the color is inversely proportional to the concentration of mycotoxins in the sample or standard. A stop solution is then added which changes the color from blue to yellow. The microwells are measured optically by a microplate reader with an absorbance filter. The optical densities of the samples are compared to the OD’s of the standards and an interpretative result is determined.
In the described study AgraQuant® Deoxynivalenol Assay 0.25/5.0, AgraQuant® Zearalenone Assay 40/100 and AgraQuant® Total Fumonisin Assay 0.25/5.0 were used. The first number in the test kits name refer to the limit of quantification [µg/kg], the second number to the upper limit of the working range [µg/kg] of the respective kit.
Deoxynivalenol
For analyzing DON the AgraQuant® DON Assay was used. This kit was validated by the manufacturer for the use in corn, wheat, barley and many other commodities. 20g of representative sample was weighed into a glass char, 100ml of distilled water was added. The sample was extracted by shaking for 1 hour at 180rpm (rotations per minute) on a GFL shaker. After performing the ELISA test according to described procedure, micro wells were measured by using an absorbance filter of 450 nm (OD450).
Zearalenone
For analyzing ZON the AgraQuant® ZON Assay was used. This kit was validated by the manufacturer for the use in corn, wheat, barley and many other commodities ZON was extracted from 20g representative sample by shaking with 100ml of 70% methanol and 4g NaCl for 1 hour at 180rpm (rotations per minute) on a GFL shaker. After performing the ELISA test according to the described procedure, the micro wells were measured by using an absorbance filter of 450 nm and a differential filter of 630nm.
Total Fumonisin
For analyzing FUM the AgraQuant® Total Fumonisin Assay was used. This test was validated by the manufacturer for maize, wheat, rice and other commodities. For extraction, 100ml of 70% methanol were added to 20g of the sample and shaken for 1 hour as described for ZON or DON previously. After performing the test according to the manufacturer’s guidelines, the micro wells were measured by using an absorbance filter of 450 nm and a differential filter of 630nm.
Results
Authors note
Results of mycotoxin occurrence are discussed in detail for corn, wheat and barley, which is not possible for rye, rice and soybean meal due to the limited number of samples received.
Mycotoxin occurrence in Spain with regard to sourcing regions:
90 grain samples were collected and analyzed for ZON and DON content resulting in 180 analyses. In order to get more information about the co-occurrence of mycotoxins produced by different types of fungi the 20 samples showing the highest contamination with DON were analyzed for total fumonisin additionally. Table 3 gives a descriptive overview of mycotoxin contamination levels with respect to sourcing regions.
Central-North Spain
A total number of 51 analyses were done on samples deriving from Central-north Spain (57%). Occurrence of mycotoxins in the area central-north Spain was 55% and 6% for DON and ZON respectively. DON was the prevalent mycotoxin in this area (55%) with the highest level detected in a corn sample contaminated with 907 µg/kg. Of the 51 samples derived from Central-North Spain, 10 were subjected to FUM analysis. Total FUM was detected in 40% of these samples. 8542 µg/kg in a corn sample was the maximum analyzed level. The highest ZON level was found in a corn sample at 180 µg/kg.
North-East Spain
At first sight, contamination profile of samples from north-east Spain seems to be very similar to the profile of samples from central region, but they differ in one major aspect. 39 samples derived from this area were analyzed, with 44% detected positive on DON and 8% on ZON respectively. The highest DON level detected was 1148 µg/kg in a corn sample and the highest ZON level was 171 µg/kg. Thus, regarding ZON and DON contamination the profile looks similar to the one of central-north Spain. The major difference occurs in the FUM contamination levels. FUM had a rather high prevalence rate in samples from North-East Spain. 70% of the 10 analyzed samples were detected positive, which is rather highe compared to the country average (55%). The highest contamination level in samples from this area was 5217 µg/kg.
Mycotoxin occurrence in Spain according to commodities
Table 2 gives an overview of contamination of commodities tested, stating the total number of each raw material tested, the number of positives, the mean of positive samples and the highest detected level per commodity. Due to the low number of rice and soybean samples and for better illustration data of the following grains are discussed in the paper in detail: corn, wheat and barley.
The other commodities are summarized in the group “other commodities”.
Corn
As the most commonly used feed ingredient, corn resulted in being the most affected commodity. Corn accounts for 51% of all the commodities tested, resulting in being positive for DON, ZON and FUM. DON was found in 84% of the corn samples (high 1148 µg/kg, average 499 µg/kg). ZON and FUM were also present in the corn samples. With 6 corn samples tested positive on ZON (13%), all positive ZON results were found in corn samples. Of the 46 corn samples, only 17 were subjected to FUM analysis. From the analyzed samples, more than 64% of the corn samples were tested to be positive (high 8542 µg/kg, average 3716 µg/kg).
Barley
A total of 17 barley samples were tested on the occurrence of ZON and DON, with all of them below LOD.
Wheat
20 wheat samples were analyzed and contamination in wheat was at relatively low levels. No ZON were found in any sample tested. DON accounted for 15% of the contamination (high 638 µg/kg, average 463 µg/kg). Only one sample was analyzed on FUM (tested negative), hence the FUM occurrence in wheat may not be conclusive.
Other commodities
Rice, rye and soy samples are the commodities summarized in this group and analyses show prevalence for DON with 43% (high 844 µg/kg, average 596 µg/kg). ZON and FUM were not detected in these samples.
Of the seven samples only the 2 soy samples were subjected to FUM analysis. Though both were tested positive for DON no FUM contamination was observed.
Conclusion and discussion of strategies to prevent and counteract mycotoxins
Incidence and impacts of mycotoxins
Given the vast diversity of commodities that may be infected by fungi, it is important to understand that the presence of specific fungi does not necessarily imply that a fungal toxin is present and that it is difficult (if not impossible) to predict mycotoxin occurrence. Thus it is pertinent to analyze mycotoxins in all cases as far as possible, considering factors like certain commodities, susceptibility of certain animal species, and geographical factors accordingly.
In summary 50% of all samples were tested positive on the occurrence of DON and 7% tested positive on ZON respectively. Selecting the 10 highest contaminated DON samples from each region FUM analysis was conducted additionally. 55% of the additionally analyzed samples were tested positive for FUM.
No correlation could be found between high DON and simultaneous high FUM contamination. Although both mycotoxins are produced by Fusarium fungi, the Fusarium species differ. DON is produced mainly by F.culmorum and F.graminearum, whereas F.moniliforme and F.proliferatum are responsible for the FUM production (BOTTALICO 1998, CAST 2003). The highest FUM contamination (8.542 µg/kg) was found in a corn sample with comparatively low DON contamination (537 µg/kg). ON the other hand, highest DON contamination (1.148 µg/kg) was found in a sample that showed relatively low FUM contamination (3.350 µg/kg). Both Soy samples resulted positive on DON but no FUM were detected.
The economic costs of mycotoxins are impossible to be accurately determined, but the US Food and Drug Administration (FDA) give estimations based on a computer model: in the US only the mean economic annual costs of crop losses from the mycotoxins aflatoxins, fumonisins, and deoxynivalenol, are estimated to be USD 932million (CAST, 2003).
Preharvest strategies
Mycotoxins occur despite the most strenuous efforts on prevention. Prevention of fungal infections during plant growth, harvest, storage and distribution would be the most rational and efficient way to avoid mycotoxins in agricultural commodities and thus all their negative impacts. Common practical measures include for instance planting of more resistant grains, selection of high quality seeds, avoiding of high plant densities, balanced fertilisation, preventive management towards insect infestations as well as a suitable management of crop residues that often provide the primary inoculum of mycotoxigenic fungi. During harvest the appropriate date, suitable harvesting equipments and careful harvesting procedures to minimize crop damage as well as the removal of damaged portions of crops and high moisture plant parts can possibly reduce mould infections and subsequent mycotoxin production. Storage without delay in good storage facilities under moisture-, temperature-, humidity- and insect-control and the addition of certain antifungal agents may also diminish fungal growth but cannot detoxify already mycotoxin-containing feedstuffs.
However, contamination of agricultural products with mycotoxins occurs despite the most strenuous efforts on prevention. The economic impacts are felt by crop- and animal producers as well as by food- and feed processors.
Postharvest strategies
Costs and limitations of physical and chemical treatments of feed prompted the search for other solutions concerning the mycotoxin hazard. Consequently, techniques were investigated based on deactivation of mycotoxins directly in the gastrointestinal tract of animals (in vivo).
Up to now, the most widely investigated method in this field is the addition of chemisorbents with the capacity to tightly bind and immobilize mycotoxins in the gastrointestinal tract of animals, resulting in a major reduction of toxin bio-availability. In several studies, hydrated sodium calcium aluminosilicates (HSCAS) have proven to be the most promising adsorbents. However, while good and scientifically explained results were obtained for counteracting aflatoxins (Ramos and Hernandez, 1996; Scott et al., 1998), adsorption of other mycotoxins was limited (e.g. zearalenone, ochratoxin A) or even failed (e.g. trichothecenes, like deoxynivalenol) under field conditions (Friend et al., 1984; Kubena et al., 1990; Huff et al., 1991; Kubena et al., 1993; Ramos et al., 1996).
The extensive use of adsorbents in the livestock industry has led to the introduction of a wide range of new products on the market, most of them claiming high in vitro mycotoxin adsorption capacity. However, adsorbents that may appear effective in vitro do not necessarily retain their efficacy when tested in vivo (Avantaggiato et al., 2005).
In the case of less- and non-adsorbable mycotoxins new paths have to be trodden. However, enzymatic or microbial degradation of mycotoxins (“biotrans-formation”) has already been a subject of research for more than 30 years (Binder et al., 2000; Kollarczik et al., 1994; He at al., 1992; Yoshizawa et al., 1983 and 1994). A great deal of respective literature is available concerning the biotransformation of trichothecenes, as these mycotoxins actually belong to the agriculturally most important toxins worldwide. By now it is well known that the 12,13-epoxide ring of these compounds is responsible for their toxic activity and that a reductive de-epoxidation caused by specific enzymes (de-epoxidases) entails a significant loss of toxicity (figure 1). However, Binder et al. were the first to develop a trichothecene-deactivating feed additive based on live microbes (Eubacterium BBSH 797).
Toxicity of zearalenone (ZEA) is based on its structural similarity to the female hormone estrogen. Like estrogen, ZEA can be bound to the estrogen-receptor, finally resulting in characteristic symptoms called “hyperestrogenism” (= fertility problems).
In the course of a several-year research project, the feed additive Biomin® MTV was developed and patented. The live yeast species was named Trichosporon mycotoxinivorans after its unique property to “eat” and thus detoxify both, zearalenone and ochratoxin A (lat.: vorans = eating, devouring).
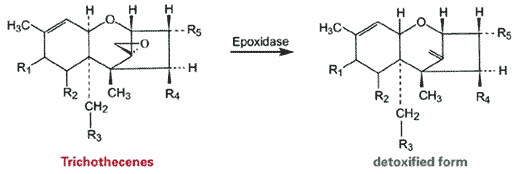 |
| Fig. 1:Detoxification of trichothecenes. The 12,13 epoxide ring of trichothecenes is able to react with DNA and consequently impairs protein biosynthesis in animals. As soon as the reactive epoxide ring is removed by specific enzymes, non-toxic metabolites are obtained that can no longer interfere with protein biosynthesis. |
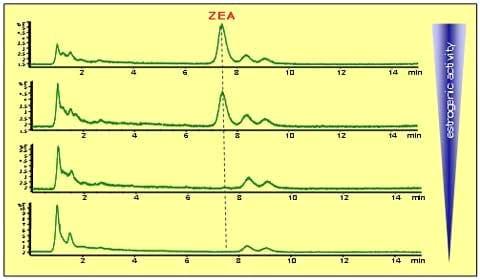 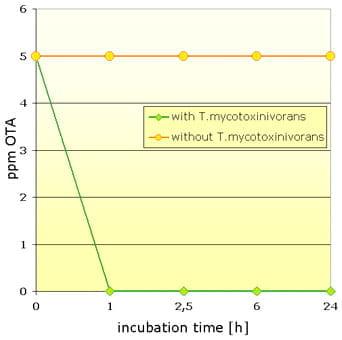 |
| Fig.2: Left graph: Full degradation of 1 mg/kg ZEA (retention time 7.45 min) by T. mycotoxinivorans. Right graph: OTA (5 mg/kg)-detoxification within £ 1 hour. |
| Conclusions |
| For many reasons it is not possible to totally avoid mycotoxin-contaminations in feed. From the data it appears, that commodities used in the Spanish feed industry, are at risk from mycotoxin contamination. A lot of research has been done to adsorb or deactivate these toxins in the intestinal tract of animals with products that are directly mixed into the feed. It turned out that some mycotoxins, like trichothecenes, cannot be adsorbed sufficiently. Thus, enzymatic biotransformation to metabolites without pathogenic activity is the only way to avoid their negative effects on animals. Only the combination of different strategies will finally lead to success. |
| Table 1: Occurrence and highest levels of mycotoxins detected based on commodity and type. | |||||
| Mycotoxin | Sample size | Percent positive (%) | Average of positive (µg/kg) | Highest level detected (µg/kg) | Commodity found |
Deoxynivalenol | 90 | 50 | 444 | 1148 | Corn |
Zearalenon | 90 | 7 | 104 | 180 | Corn |
Fumonisin B1 | 20 | 55 | 3350 | 8542 | Corn |
| Table 2: Incidence of mycotoxin contamination in feed commodity sourced in Spain. | ||||||
| DON | ZON | FUM | ||||
| Total Nr.a | Nr. pos.b | Total Nr.a | Nr. pos.b | Total Nr.a | Nr. pos.b | |
| Mean/Med.c | Max. lev.d | Mean/Med.c | Max. lev.d | Mean/Med.c | Max. lev.d | |
| 46 | 39 | 46 | 6 | 17 | 11 | |
| Corn | 499/444 | 1148 | 112/104 | 180 | 3716/3350 | 8542 |
| Wheat | 20 | 3 | 20 | 0 | 1 | 0 |
| 463/414 | 638 | 0/0 | 0 | 0/0 | 0 | |
| Barley | 17 | 0 | 17 | 0 | 0 | 0 |
| 0/0 | 0 | 0/0 | 0 | 0/0 | 0 | |
| Others | 7 | 3 | 7 | 0 | 2 | 0 |
| 596/624 | 844 | 0/0 | 0 | 0/0 | 0 | |
| DON…Deoxinivalenol; ZON…Zearalenon; FUM…Fumonisin B1 | ||||||
| aTotal number of samples analyzed bNumber of samples tested positive cArithmetic mean of all samples tested/median of positives in µg/kg dMaximum level detected in µg/kg n.d.=non detect according to LOQ as indicated in table 1 |
Table 3: Incidence of mycotoxin contamination in Spain. | ||||||
| DON | ZON | FUM | ||||
| Total Nr.a | Nr. pos.b | Total Nr.a | Nr. pos.b | Total Nr.a | Nr. pos.b | |
| Mean/Med.c | Max. lev.d | Mean/Med.c | Max. lev.d | Mean/Med.c | Max. lev.d | |
| 51 | 28 | 51 | 3 | 10 | 4 | |
| Central-North Spain | 469/440 | 907 | 106/90 | 180 | 4302/3989 | 8542 |
| North-East Spain | 39 | 17 | 39 | 3 | 10 | 7 |
| 560/460 | 1148 | 118/117 | 171 | 3381/3350 | 5217 | |
| Spain total | 90 | 45 | 90 | 6 | 20 | 11 |
| 503/444 | 1148 | 112/104 | 180 | 3716/3350 | 8542 | |
| DON…Deoxinivalenol; ZON…Zearalenon; FUM…Fumonisin B1 | ||||||
| aTotal number of samples analyzed bNumber of samples tested positive cArithmetic mean of all samples tested/median of positives in ìg/kg dMaximum level detected in ìg/kg n.d.=non detect according to LOQ as indicated in table 1 |
References:
Avantaggiato, G., Solfrizzo, M., Visconti, A. 2005. Recent advances on the use of adsorbent materials for detoxification of Fusarium mycotoxins. Food Addit. Contam., 22 (4), 379-388.
Binder, E. M., Heidler, D., Schatzmayr, G., Thimm, N., Fuchs, E., Schuh, M., Krska, R., and Binder, J., 2000, Microbial detoxification of mycotoxins in animal feed, Proceedings of The 10th International IUPAC Symposium on Mycotoxins and Phycotoxins, Brasil.
Bottalico, A. 1998. Fusarium diseases of cereals: species complex and related mycotoxin profiles, in Europe. Journal of plant Pathology, 80(2). Edition ETS Pisa, p. 85-103
CAST Report. 2003. Mycotoxins: Risks in Plant, Animal, and Human Systems. In: J.L. Richard, G.A. Payne (Eds). Council for Agricultural Science and Technology Task Force Report No. 139, Ames, Iowa, USA. ISBN 1-887383-22-0.
Erber, E. and Binder, E. M. 2004. Managing the risk of mycotoxins in modern feed production. The 5th Korea Feed Ingredient Association International Symposium, Seoul, Korea, July 16 2004. Proceedings Book 21-45.
Fink-Gremmels, J., 1999, Mycotoxins: their impact on human and animal health, Veterinary Quarterly 21: 115-120.
Friend, D.W., Trenholm, H.L, Young, J.C., Thompson, B.K., and Hartin, K.E., 1984, Effect of adding potential vomitoxin (deoxynivalenol) detoxicants of a F. graminearum inoculated corn supplement to wheat diets fed to pigs, Canadian Journal of Animal Science 64: 733-741.
Huff, W.E, Kubena, L.F., Harvey, R.B., and Phillips, T.D., 1992, Efficacy of Hydrated Sodium Calcium Aluminosilicate to Reduce the Individual and Combined Toxicity of Aflatoxin and Ochratoxin A, Poultry Science 71: 64-69.
He, P., Young, L.G., and Forsberg, C., 1992, Microbial Transformation of Deoxynivalenol (Vomitoxin), Applied and Environmental Microbiology 58 (12): 3857-3863.
Kollarczik, B., Gareis, M., Hanelt, M., 1994, In Vitro Transformation of the Fusarium Mycotoxins Deoxynivalenol and Zearalenone by the Normal Gut Microflora of Pigs, Natural Toxins 2: 105-110.
Kubena, L.F., Harvey, R.B., Huff, W.E., Corrier, D.E., Phillips, T.D., and Rottinghaus, G.E., 1990, Efficacy of a Hydrated Sodium Calcium Aluminosilicate to Reduce the Toxicity of Aflatoxin and T-2 Toxin, Poultry Science 69: 1078-1086.
Kubena, L.F., Harvey, R.B., Huff, W.E., Elissalde, A.G., Yersin, A.G, Phillips, T.D., and Rottinghaus, G.E., 1993, Efficacy of a Hydrated Sodium Calcium Aluminosilicate to Reduce the Toxicity of Aflatoxin and Diacetoxyscirpenol, Poultry Science 72: 51-59.
Ramos, A.J, and Herandez, E., 1996, Prevention of aflatoxicosis in farm animals by means of hydrated sodium calcium aluminosilicate addition to feedstuffs: a review, Animal Feed Science Technology 65: 197-206.
Richard, J. 2000. Sampling and sample preparation for mycotoxin analysis. Romer Labs Guide to Mycotoxins, Vol 2. Romer Labs. Inc., 1301 Stylemaster Drive, Union, MO 63084-1156, USA.
Scott, P.M., 1998, Industrial and farm detoxification processes for mycotoxins, Revue Médicine Véterinaire, 149 (6): 543-548
Visconti, A. 1998. Problems associated with Fusarium mycotoxins in cereals. In: Zuxun J, Quan L, Yongsheng L, Zianchang T, Lianghua G, editors. Stored product protection. Chengdu:
Sichuan. p 173–186.
Yoshizawa, T., Takeda, H., and Oli, T., 1983, Structure of a Novel Metabolite from Deoxynivalenol, a Trichothecene Mycotoxin, in Animals, Agrigultural Biological Chemistry 47 (9): 2133-2135.
Yoshizawa, T., Yamashita, A., and Luo, Y., 1994, Fumonisin Occurrence in Corn from High- and Low-Risk Areas for Human Esophageal Cancer in China, Applied and Environmental Microbiology 60 (5): 1626-1629.
Authors:A. Hainz a, E. Pichler b, J. Handl c, U. Hofstetter a
a Biomin GmbH, Industriestrasse 21, 3130 Herzogenburg, Austria
b Quantas Analytik GmbH – Europe, Technopark 1, 3430 Tulln, Austria
c Erber AG, Industriestrasse 21, 3130 Herzogenburg, Austria











