Mycotoxins, cattle and dairy food products
The top ten most frequently-asked questions about mycotoxins, cattle and dairy food products
Introduction
Mycotoxins are toxic secondary metabolites produced by fungi (molds). Secondary metabolites are chemicals produced by the fungus that are not essential for growth. Mycotoxins are chemically diverse, represent a variety of chemical families, and range in molecular weight from c. 200 to 500. A practical definition of a mycotoxin is a fungal metabolite that causes an undesirable effect in exposed animals. The undesirable effect or disease caused by a mycotoxin is a mycotoxicosis (Nelson et al., 1993). Exposure is generally through consumption of contaminated feedstuffs, although dermal contact or inhalation of certain mycotoxins can also cause undesirable responses. Mycotoxins exhibit broad and variable biological effects in animals. Mycotoxins can cause damage to organ systems, reduce production and reproduction and increase disease by reducing immunity. Some mycotoxins are carcinogens. Some target the liver, the kidney, the digestive tract or the reproductive system. Symptoms are wide ranging including decreased feed consumption, poor feed utilization, weight loss, reduced performance, estrogenic effects, vomiting, diarrhea, nervous disorders, tissue necrosis, hemorrhage, tumors, abortions and death.
Do molds cause animal problems or do molds simply produce mycotoxins for that purpose?
There is no confirmed reason for the existence of mycotoxins. Most theories suggest that mycotoxins exist to protect or enhance the existence of the fungus. Recent speculation is that mycotoxins increase the ability of the mycotoxin-producing fungus to cause a plant disease, thus helping to create an environment conducive for growth of the fungus (CAST, 2003). In experiments, Fusarium graminearum and F. verticillioides were genetically altered so that they would not produce trichothecenes or fumonisins, respectively. Results were mixed, demonstrating that trichothecenes play an important role in wheat head blight and corn ear rot caused by F. graminearum (Desjardins and Hohn, 1997; Harris et al., 1999), but that fumonisins are not required for corn ear rot caused by F. verticillioides (Desjardins et al., 2002).
It is also possible that immune suppression in animals by certain mycotoxins is in fact a mechanism to allow infectivity by the fungus. Some fungi are infectious pathogenic agents that cause a mycosis (fungal infection) that has a detrimental effect on the host animal. Aspergillus fumigatus is thought to be a fairly common mold in both hay (Shadmi et al., 1974) and silage (Cole et al., 1977). Aspergillus fumigatus has been proposed as the pathogenic agent associated with mycotic hemorrhagic bowel syndrome in dairy cattle, which has also been attributed to Clostridial infections and other factors (Puntenney et al., 2003). Such mycoses occur in immunosuppressed animals. Dairy cows are immune suppressed in early lactation. Aspergillus fumigatus also produces a mycotoxin, gliotoxin, which is an immune suppressant. It is possible that immune suppression by gliotoxin is a mechanism that allows infectivity by the fungus. Gliotoxin was found in peritoneal lavages from mice innoculated and infected with A. fumigatus (Eichner et al., 1988). Gliotoxin has also been found in the udder of cows naturally infected with A. fumigatus, while other known mycotoxins produced by this fungus were absent (Bauer et al., 1989). Interactions with trichothecene mycotoxins may also be a factor in occurrence of a mycosis because reductions in cellular immunity can reduce resistance to a mycosis. Niyo et al. (1988a, b), showed that rabbits exposed to T-2 toxin had a decrease in phagocytosis of Aspergillus fumigatus conidia by alveolar macrophages and an increase in severity of experimental aspergillosis. Richard (1991) has suggested that medical mycologists should consider this aspect of infections caused by any toxigenic fungus, especially those that produce immunosuppressive compounds. Fungal pathogens include Aspergillus fumigatus, Candida albicans, Candida vaginitis and certain species of Fusarium.
Fungi are deterioration organisms. Therefore, feedstuffs on which they grow are deteriorated and have an altered nutritional value including decreases in fat, protein and carbohydrates, which can affect performance and health (DiConstanzo et al., 1995). Cook and Wu (1991) itemized some of the nutritional changes in feeds occurring with mold growth including a decrease in lysine and thiamin and an increase in fiber. Some of the interactions of mycotoxins with nutrients have been reviewed (Schaeffer and Hamilton, 1991).
How many mycotoxins exist – how frequently are they found? Hundreds of mycotoxins have been identified, but other than the major mycotoxins, most have not been extensively researched and even fewer have good methods of analysis available. The major classes of mycotoxins are aflatoxins, zearalenone, trichothecenes, fumonisins, ochratoxin A and the ergot alkaloids. These mycotoxins are the more likely causes of mycotoxicoses in dairy cattle and other domestic animals because they occur more frequently and have the potency to cause toxicities. However, there are many reports of mycotoxicoses that have occurred as a result of those mycotoxins that are categorized as minor in importance (CAST, 2003; Lacey, 1991).
Riley (1998) put forth an argument that only a small proportion of mycotoxins have yet been identified. One factor that supports this idea is the high rate of discovery of new mycotoxins. To further support this idea, Riley (1998) cited the following facts, which we have taken the liberty to condense. Riley (1998) noted that Hawksworth (1991) estimated that there may be as many as 1.5 million fungal species in the world, but only about 69,000 are currently identified. Turner (1978) and Turner and Aldridge (1983), catalogued 3200 secondary metabolites produced by 1600 fungal species, or two secondary metabolites per fungal species. Cole and Cox (1981) classified 10% of the fungal secondary metabolites as mycotoxins. If there are indeed 1.5 million fungal species and if each produces two secondary metabolites and if 10% of secondary metabolites are mycotoxins, there are potentially 300,000 mycotoxins. More conservatively, if there are 100,000 fungal species producing 200,000 secondary metabolites, there may be 20,000 mycotoxins in nature. Potentially, many more mycotoxins exist than have been identified. The potentially large number of unidentified mycotoxins and the fact that commercial laboratory analyses are not available for many of the identified mycotoxins suggests that a mycotoxicosis can occur without any possibility of identifying the mycotoxin, or all of the mycotoxins, that may be interacting to produce the mycotoxicosis.
The frequency of the occurrence of mycotoxins and the proportion of feeds that are contaminated indicate that animal exposure is high. The FAO has suggested that 25% of the world’s crops are affected (CAST, 1989). Certainly raw agricultural products are more likely to be contaminated than the human food supply. Mycotoxins are present worldwide with some geographical differences mainly resulting from climatic differences. There are differences in mycotoxins by type of feedstuff. Occurrence and concentrations are variable by year, which is expected because of the annual variation in weather conditions and plant stresses known to affect mycotoxin formation (Coulumbe, 1993). Summaries of surveys showing the incidence and concentrations of mycotoxins in various feedstuffs have been published (CAST, 2003; Wood, 1992; Wood and Trucksess, 1998). Feed samples submitted by North Carolina farmers over a 13 year period (Table 1) indicate that mycotoxins in feeds occur commonly at unsuitable concentrations (Whitlow et al., 1998). It can be concluded that mycotoxins occur frequently in a variety of feedstuffs and are routinely fed to animals.
Table 1. Mycotoxin occurrence by concentration in all feeds submitted for analysis by North Carolina farmers over 13 years.
| Aflatoxin | Deoxynivalenol | Fumonisin | T-2 Toxin | Zearalenone | |
| Number of samples | 3266 | 5053 | 822 | 5136 | 4563 |
| Low, % of samples below (concentration) | 6.4 (<20 ppb) | 18.2 (<500 ppb) | 32.6 (<5000 ppb) | 1.5 (<100 ppb) | 7.1 (<300 ppb) |
| High, % of samples above (concentration) | 4.0 (>19 ppb) | 28.2 (>499 ppb) | 9.4 (>4999 ppb) | 6.6 (>99 ppb) | 8.3 (>299 ppb) |
What conditions support mold growth and mycotoxin formation?
Perhaps the major mycotoxin-producing fungal genera, in terms of research in the United States, are Aspergillus, Fusarium, and Penicillium. Many species of these fungi produce mycotoxins in a variety of feedstuffs. Claviceps spp. particularly in small grains and Epichloe and Neotyphodium in fescue grass all produce ergot alkaloids. These fungi and their mycotoxins are also a concern, but have a more specific host-fungal relationship than do Aspergillus, Fusarium, and Penicillium fungi. Molds are fungi that grow in multicellular colonies, as compared with yeasts that are single cellular fungi. Molds can grow and mycotoxins can be produced pre-harvest or during storage, transport, processing, or feeding. Mold growth and mycotoxin production are related to weather extremes (causing plant stress or excess hydration of stored feedstuffs), to inadequate storage practices, to low feedstuff quality, and to faulty feeding conditions. In general, environmental conditions - heat, water, and insect damage - cause plant stress and predispose plants in the field to mycotoxin contamination. Because feedstuffs can be contaminated pre-harvest, control of additional mold growth and mycotoxin formation is dependent on storage management. After harvest, temperature, moisture content, and insect activity are the major factors influencing mycotoxin contamination of feed grains and foods (Coulumbe, 1993).
Molds grow over a temperature range of 10-40°C (50-104°F), a pH range of 4 to 8, and above 0.7 aw (equilibrium relative humidity expressed as a decimal instead of a percentage). Mold can grow on feeds containing more than 12-13% moisture. In wet feeds such as silage, higher moisture levels help exclude air and molds grow only if oxygen is available. The conditions most suitable for mold growth may not be the optimum conditions for mycotoxin formation in the laboratory. For example, the Fusarium molds associated with alimentary toxic aleukia have been reported to grow prolifically at 25-30°C without producing much mycotoxin, but at near-freezing temperatures large quantities of mycotoxins were produced with minimal mold growth (Joffe, 1986). Field applications of fungicides may reduce mold growth, in turn reducing the production of mycotoxins. However, the stress or shock of the fungicide to the mold organism may cause increased mycotoxin production (Boyacioglu et al., 1992; Gareis and Ceynowa, 1994).
Aspergillus species normally grow at lower water activities and at higher temperatures than do the Fusarium species. Therefore, Aspergillus flavus and aflatoxin in corn are favored by the heat and drought stress associated with warmer climates. Penicillium species grow at relatively low water activities and low temperatures and are widespread in occurrence. Penicillium molds are more common in storage than in preharvest, but can grow in the field under very wet conditions. Because both Aspergillus and Penicillium can grow at low water activities, they are considered storage fungi (Christensen et al., 1977).
Growth of A. flavus can occur at 86-87% equilibrium relative humidity (RH) (Davis and Diener, 1983). Field infection of corn with A. flavus (Wicklow, 1983) is expected when temperatures, including nighttime temperatures, are high and there is drought stress. Growth conditions in the southern US result in routine aflatoxin contamination of crops, but aflatoxin can be found in crops grown in other regions in years when weather conditions are conducive. For example, 8% of samples of midwestern US corn grain from the 1988 drought season contained aflatoxin (Russell et al., 1991). Corn is susceptible to A. flavus infection via the silks (Marsh and Payne, 1984) and stress conditions at the time of anthesis (pollination) lead to preharvest aflatoxin contamination in corn. A. flavus spores as inoculum are plentiful at this time. In North Carolina, insect activity appears less important in the events leading to aflatoxin contamination of corn than it appears to be in Georgia (Payne, 1983). Aflatoxin is a greater problem in cottonseed grown in the southwestern US than in the southeastern US (Ashworth et al., 1969). The complex effects of relative humidity, temperature, precipitation, and their daily variations may interact to produce conditions conducive to A. flavus infection and aflatoxin production in the Southwest (Ashworth et al., 1969). Early harvest and a decrease in late-season irrigation may reduce contamination (Russell et al., 1976). Experimentally, the use of spores of nontoxigenic A. flavus isolates in southwestern cotton fields has resulted in greatly reduced aflatoxin levels in cottonseed (Cotty et al., 1994). Improperly stored cottonseeds are susceptible to mycotoxin contamination if mold activity is allowed.
The Fusarium species are generally considered to be field fungi and were thought to proliferate before harvest (Christensen et al., 1977). However, Fusarium species may also grow and produce mycotoxins under certain storage conditions. In corn, Fusarium molds are associated with ear rot and stalk rot, and in small grains, they are associated with diseases such as head blight or commonly referred to as scab (Tuite et al., 1974). Fusarium is associated with excessive moisture at flowering. In corn, Fusarium diseases are more commonly associated with a cool wet growing season, with insect damage, warm conditions at silking, and wet conditions late in the growing season (Trenholm et al., 1988).
Of the Fusarium species F. graminearum is a major producer of deoxynivalenol (DON) and zearalenone (ZEN), but other species of Fusarium also produce DON and ZEN, as well as other mycotoxins (Christensen et al., 1988; Marasas et al., 1984). Conditions exacerbating ZEN accumulation in corn include weather that holds moisture content at 22- 25%, or delayed harvest (Abbas et al., 1988). Zearalenone has been reported to occur in corn, other grains, and silage in many areas of the world. Weathered soybeans have also been reported to be contaminated with ZEN (Hagler et al., 1989). ZEN is also found in wheat, barley, oats, sorghum, sesame seed, hay, and silages. DON occurs in cereal grains worldwide and can increase in stored grain with kernel moisture contents of 22–25%. Minimum tillage and no tillage production are believed to increase the amount of disease in small grains and corn/wheat rotations because of increased inoculum survival on crop residue (Trenholm et al., 1988). T-2 toxin is produced primarily by F. sporotrichioides and F. poae, but is also produced by other species of Fusarium (Marasas et al., 1984). T-2 (and DAS) is often found in barley, wheat, millet, safflower seed, and in mixed feeds.
How is a mycotoxicosis diagnosed?
Hamilton (1978) presented an interpretation of the application of Koch’s postulates to mycotoxins. The postulates as modified are:
1. Find the mycotoxin in suspect substrate from the toxicosis outbreak.
2. Find in the substrate a fungus that produces the toxin.
3. Induce the toxicosis in experimental animals by ingesting or contacting the toxin.
Once the mycotoxicosis is established as a disease entity, it is no longer necessary to repeat the process. Recognition of the mycotoxicosis symptoms and mycotoxin presence in feed provide an adequate basis for diagnosis. If symptoms unique to that mycotoxin are observed, then it is not necessary to determine that the mycotoxin is in the feed.
Mycotoxins result in a progression and diversity of symptoms that can be confusing and can make diagnosis difficult (Hesseltine, 1986; Schiefer, 1990). Symptoms from field cases can be different from those observed under controlled experimental conditions because in field cases there may be multiple mycotoxins, variable dosages at irregular intervals, uncontrolled environments, and various interacting stress factors. Diagnosis is complicated by a lack of research, by a lack of feed analyses, by numerous possible mycotoxins, by nonspecific symptoms, and by immunosuppression resulting in opportunistic diseases that produce confounding symptoms. Therefore, a definitive diagnosis of a mycotoxicosis is difficult from general symptoms, specific tissue damage, or even feed analyses. However, experience with mycotoxin-affected herds greatly increases the probability of recognizing a mycotoxicosis. A process of elimination of other factors, coupled with feed analyses and unique symptoms can help identify a mycotoxicosis. Another practice helpful in diagnosis is an observation of positive responses or alleviation of symptoms after the use of products known to be effective in reducing mycotoxin exposure to animals. Examples of such products are mold inhibitors and mycotoxin sequestering agents. Regardless of the difficulty of diagnosis, mycotoxins should be considered as a possible cause of production and health problems when pertinent symptoms exist and problems are not directly attributable to other typical causes (Schiefer, 1990).
How do mycotoxins affect dairy cows?
Mycotoxins can increase incidence of disease and reduce production efficiency. Some of the gross effects of mycotoxins can include:
1) intake reduction or feed refusal,
2) reduction in nutrient absorption and metabolism,
3) digestive disorders including hemorrhage and necrosis,
4) tissue and organ damage,
5) gangrene of the extremities,
6) endocrine effects,
7) reproductive disorders, embryonic death, abortions,
8) nervous disorders, tremors, uncoordination,
9) suppression of the immune system, and
10) death.
Symptoms will be dependent on the mycotoxins present. In the field, animals experiencing a mycotoxicosis may exhibit a few or many symptoms. They may simply be unthrifty, with a rough or dull hair coat, have an undernourished appearance, impaired reproduction, and(or) a mixed infectious disease profile. Some of the symptoms observed with a mycotoxicosis may be secondary, resulting from an opportunistic disease that is present because of immune suppression caused by the mycotoxin exposure.
Toxicity occurs at the cellular level. Aflatoxin causes DNA changes, cell deregulation, cellular changes and death. Deoxynivalenol inhibits protein synthesis resulting in disruption of cytokine regulation, altered cell proliferation and cell death. T-2 toxin inhibits protein synthesis with subsequent cell death. Fumonisin alters enzyme activity, which disrupts lipid metabolism resulting in cell deregulation and cell death. Zearalenone binds with cytosolic estrogen receptors causing an estrogenic response and altering hormonal control (Riley and Norred, 1996).
AFLATOXINS
Aflatoxins are a family of extremely toxic, mutagenic, and carcinogenic compounds produced by Aspergillus flavus and A. parasiticus (Deiner et a1., 1987; Kurtzman et al., 1987). Toxigenic A. flavus isolates produce aflatoxins B1, and B2 and toxigenic A. parasiticus isolates produce aflatoxins B1, B2, G1, and G2 (Cotty et al., 1994).
Symptoms of acute aflatoxicosis in mammals include inappetance, lethargy, ataxia, rough hair coat, and pale, enlarged fatty livers. Symptoms of chronic aflatoxin exposure include reduced feed efficiency and milk production, icterus, and decreased appetite (Nibbelink, 1986). Reduced growth rate may be the only clue for chronic aflatoxicosis and other mycotoxicoses (Raisbeck et al., 1991; Pier, 1992). The mechanism by which aflatoxins reduce growth rate is probably related to disturbances in protein, carbohydrate and lipid metabolism (Cheeke and Shull, 1985).
Depending on interactions with other factors, aflatoxin concentrations as low as 100 ppb may be toxic to dairy and beef cattle, however the toxic level is generally considered to be between 300 to 700 ppb. Garrett et al. (1968) showed an effect on weight gain and intake with diets containing 700 ppb aflatoxin, but if increases in liver weights are used as the criteria for toxicity, then 100 ppb would be considered toxic to beef cattle. Guthrie (1979) showed a decline in reproductive efficiency when lactating dairy cattle in a field situation were consuming 120 ppb aflatoxin. When cows were changed to an aflatoxin-free diet, milk production increased over 25%. Patterson and Anderson (1982) and Masri et al. (1969) also suggest that 100 ppb may reduce milk production.
Aflatoxin produced from culture was shown to be more toxic to dairy cattle than pure aflatoxin added to diets (Applebaum et al., 1982). This is thought to result from other mycotoxins present in the natural culture. Under certain conditions, A. flavus also produces sclerotia, or resting bodies, which contain indole alkaloids such as aflatrem (Wicklow, 1983). Cyclopiazonic acid (CPA), a toxic indole tetramic acid, is also produced by A. flavus (CAST, 1989). The role of these and other toxins produced by A. flavus in aflatoxicoses is not known. Aflatoxin lowers resistance to diseases and interferes with vaccineinduced immunity in livestock (Diekman and Green, 1992).
The Food and Drug Administration (FDA) has established nonbinding action levels as informal guidelines for enforcement of aflatoxin control in feedstuffs (Table 2, Wood and Trucksess, 1998). Blending contaminated ingredients with uncontaminated ingredients with the purpose of reducing aflatoxin concentrations is not allowed.
Table 2. US Food and Drug Administration action levels for total aflatoxins in food and feed.
| Food or feedstuff | Concentration (ppb) |
| All products, except milk, designated for humans | 20 |
| Corn for immature animals and dairy cattle | 20 |
| Corn and peanut products for breeding beef cattle, swine, and mature poultry | 100 |
| Corn and peanut products for finishing swine (>100 lb) | 200 |
| Corn and peanut products for finishing beef cattle | 300 |
| Cottonseed meal (as a feed ingredient) | 300 |
| All other feedstuffs | 20 |
| Milk | 0.5a |
| a Wood and Trucksess, 1998. |
ZEARALENONE
Zearalenone and zearalenol are estrogenic metabolites of several species of Fusarium. Chemically, zearalenone (ZEN) is a resorcylic acid lactone which does not have actual toxicity. Zearalenone is the cause of hyperestrogenism, the estrogenic syndrome, in swine. F. graminearum is the major ZEN-producing fungus of the Fusarium species that cause corn ear and stalk rots, but other species of Fusarium produce ZEN, as well as other mycotoxins (Christensen et al., 1988).
Zearalenone is rapidly converted to α− and ßzearalenol in rumen cultures (Kiessling et al., 1984). α−Zearalenol is c. four-fold more estrogenic in rats than ZEN, while ß-zearalenol is about equal in strength to ZEN (Hagler et al., 1979). However, ZEN has been considered of less importance to ruminants. Ruminal conversion of ZEN was found to be about 30% in 48 hrs (Kallela and Vasenius, 1982). A controlled study with nonlactating cows fed up to 500 mg of ZEN (dietary concentrations of about 40 ppm ZEN) showed no obvious effects except that corpora lutea were smaller in treated cows (Weaver et al., 1986b). In a similar study with heifers receiving 250 mg of ZEN by gelatin capsule (dietary concentrations of 25-30 ppm ZEN), conception rate was depressed about 25%; otherwise, no obvious effects were noted (Weaver et al., 1986a). Several case reports have related ZEN to an estrogenic response in ruminants and sometimes included abortions as a symptom (Kallela and Ettala, 1984; Khamis et al., 1986; Mirocha et al., 1968; Mirocha et al., 1974; Roine et al., 1971). Other cattle responses may include vaginitis, vaginal secretions, poor reproductive performance and mammary gland enlargement of virgin heifers. In a field study (Coppock et al., 1990), diets with about 750 ppb ZEN and 500 ppb DON resulted in poor consumption, depressed milk production, diarrhea, and total reproductive failure. New Zealand workers (Towers et al., 1995a,b; Sprosen and Towers, 1995; Smith et al., 1995) have successfully estimated intake of ZEN and its metabolites (ZEN+M) by measuring urinary ZEN and its metabolites which include zearalanone, α- and ß-zearalenol and α- and ß-zearalanol. ZEN+M intake predicted from urinary ZEN+M was associated with reproductive disorders in sheep and dairy cattle. In sheep, ZEN+M was related to lower conception, reduced ovulation, increased twinning rates and a 10 to 20 % decline in fertility of ewes. With dairy cattle, herds with low fertility had higher levels of blood and urinary levels of ZEN+M. Individual cows within herds, examined by palpation and determined to be cycling, had lower blood ZEN+M levels than did cows that were not cycling. The reproductive problems in dairy cattle were associated with ZEN+M concentrations of about 400 ppb in the pasture samples.
TRICHOTHECENES
Trichothecenes are a family of 200-300 related compounds that apparently exert their toxicity through protein synthesis inhibition at the ribosomal level. Several species of Fusarium and related genera produce trichothecenes. T-2 toxin, diacetoxyscirpenol (DAS), and DON are commonly found in agricultural commodities (Desjardins et al., 1993). However, except for DON, it appears that most contamination with T-2 toxin and DAS occurs post-harvest. The toxic effects of trichothecenes include gastrointestinal effects such as vomiting, diarrhea, and bowel inflammation. Anemia, leukopenia, skin irritation, feed refusal, and abortion are also common. The trichothecenes, as a group, are immuno-suppressive (Sharma, 1993).
DEOXYNIVALENOL
The impact of DON on dairy cattle is not established, but clinical data show an association between DON contamination of diets and poor performance in dairy herds, but without establishing a cause and effect (Whitlow et al., 1994). DON may therefore be a marker for low-quality mycotoxin-contaminated feeds in these herds. Other case reports help substantiate an association of DON with poor performing dairy herds (Gotlieb, 1997 and Seglar, 1997). DON has been associated with reduced feed intake in nonlactating dairy cattle (Trenholm et al., 1985). There was a trend (P<0.16) for a 13% loss in 4% fat corrected milk in a study utilizing 18 midlactation dairy cows (average 19.5 kg milk), consuming diets shown to contain no common mycotoxins other than DON which was at levels of approximately 0, 2.7 and 6.5 ppm in treatment diets (Charmley et al., 1993). Noller et al. (1979) used 54 lactating dairy cows in a 3 x 3 latin square experiment with 21-day feeding periods. Gibberella zeae (F. graminearum) infected corn was used to provide estimated concentrations of 0, 1650 and 3300 ppb DON and 0, 65 and 130 ppb of ZEN in three experimental diets. While neither intake nor milk production (22.9 kg/d) were affected, cows that received contaminated grain gained significantly less weight. Conversely, Ingalls (1996) fed lactating cows diets containing 0, 3.6, 10.9 or 14.6 ppm of DON for 21 days, without an apparent effect on feed intake or milk production (30 kg/d). DiCostanzo et al. (1995), in a review of several individual studies, concluded that beef cattle and sheep can tolerate up to 21 ppm of DON without obvious deleterious effects.
The FDA had provided an advisory for DON concentrations in wheat and wheat-derived products (Table 3) (Wood and Trucksess, 1998).
T-2 TOXIN
T-2 toxin is produced primarily by F. sporotrichioides and F. poae, but is also produced by other species of Fusarium (Marasas et al., 1984). Data with cattle are limited, but the toxicity of T-2 toxin in laboratory animals is well-documented (Wannemacher et al., 1991). T-2 toxin is a very potent mycotoxin associated with gastroenteritis, intestinal hemorrhages (Petrie et al., 1977; Mirocha et al., 1976) and death (Hsu et al., 1972; Kosuri et al., 1970). T-2 toxin fed to cattle at 0.64 ppm for 20 days resulted in death and bloody feces, enteritis, and abomasal and ruminal ulcers (Pier et al., 1980). Kegl and Vanyi (1991) observed bloody diarrhea, low feed consumption, decreased milk production and absence of estrus cycles in cows exposed to T-2. Weaver et al. (1980) showed that T- 2 was associated with feed refusal and gastrointestinal lesions in a cow, but did not show a hemorrhagic syndrome. Serum immunoglobulins and certain complement proteins were lowered in calves receiving T-2 toxin (Mann et al., 1983). Gentry et al. (1984) demonstrated a reduction in white blood cell and neutrophil counts in calves. A calf intubated with T-2 developed severe depression, hindquarter ataxia, knuckling of the rear feet, listlessness and anorexia (Weaver et al., 1980).
FUMONISINS
This family of mycotoxins is produced by the species of Fusarium in the Liseola section. F. verticilloides (formerly F. moniliforme), a species that is almost ubiquitous in corn, and F. proliferatum are the main species producing high yields of fumonisins. Fumonisins B1, B2, and B3 (FB1, FB2, and FB3) are produced in fungal cultures or found in naturally contaminated corn samples (Cawood et al., 1991). Feed infected with F. verticilloides has long been associated with outbreaks of blind staggers (equine leucoencephalomalacia, ELEM) in equines (Wilson et al., 1985). Fumonisin B1 was first isolated in South Africa where F. moniliforme has long been associated with animal problems (Gelderblom et al., 1988). Fumonisin has been shown to cause leucoencephalomalacia in horses (Marasas et al., 1988), pulmonary edema in swine (Harrison et al., 1990) and hepatoxicity in rats (Gelderblom et al., 1991). Fumonisins are structurally similar to sphingosine, a component of sphingolipids. Sphingolipids are in high concentrations in myelin and in certain nerve tissues. Fumonisin toxicity is thought to result from disruption of sphingolipid biosynthesis (Riley et al., 1996). A USDA, APHIS survey of 1995 corn from Missouri, Iowa and Illinois found that 6.9% contained more than 5 ppm fumonisin B1 (Anon., 1995). Murphy et al. (1993) reported fumonisin concentrations in corn for the Iowa, Wisconsin, and Illinois crops. Incidence of contamination was greater than 60% and concentrations ranged from 0 to 37.9 ppm. Corn screenings contained c. 10 times the fumonisin content of the original corn.
Table 3. US FDA advisory levels for deoxynivalenol in wheat-derived products.1
| Product | Concentration (ppb) |
| All finished wheat products, e.g. flour, bran and germ, for human consumption | 1 |
| Grains and grain by-products destined for ruminating beef cattle and cattle in feedlots older than 4 months and for chickens (these ingredients should not exceed 50% of the diet) | 10 |
| Grains and grain by-products destined for swine (these ingredients should not exceed 20% of the diet) | 5 |
| Grains and grain by-products for all other animals (these ingredients should not exceed 40% of the diet) | 5 |
| 1 Wood and Trucksess, 1998. |
While FB1 is thought to be much less potent in ruminants than monogastrics, work by Kriek et al. (1981) suggested that fumonisin was toxic to sheep. Osweiler et al. (1993) fed young steers 15, 31 or 148 ppm fumonisin in a short term study (31 days). There were no significant effects on feed consumption or gain; however, there was a trend toward lower intake and weight gains for those fed 148 ppm. With the highest feeding level, there were mild liver lesions in calves, and the group had elevated liver enzymes indicative of liver damage. Lymphocyte blastogenesis was significantly impaired at the end of the feeding period in the group having the highest dose. Dairy cattle (Holsteins and Jerseys) fed diets containing 100 ppm fumonisin for approximately 7 days prior to freshening and for 70 days thereafter demonstrated lower milk production (6 kg/cow/day), explained primarily by reduced feed consumption. Increases in concentrations of serum enzymes suggested mild liver disease (Diaz et al., 2000). Dairy cattle may be more sensitive to fumonisin than are beef cattle, perhaps because of greater production stress.
Fumonisin has been shown to be carcinogenic in rats and mice (NTP, 1999), and has been associated with esophageal cancer in humans in China (Chu and Li, 1994) and South Africa (Rheeder et al., 1992). Therefore, fumonisin contamination has implications for human health, at least from a regulatory perspective. The FDA released guidance for fumonisin levels in human foods and animal feeds in late 2001 (Table 4).
OCHRATOXIN A
This mycotoxin is produced by species of Penicillium and Aspergillus, and is a causative agent of kidney disease in pigs that has been referred to as mycotoxin porcine nephropathy, producing symptoms including diarrhea, increased water consumption, diuresis and dehydration (Krogh, 1979). OTA is rapidly degraded in the rumen and thus thought to be of little consequence unless consumed by young pre-ruminant calves (Sreemannarayana et al., 1988).
CITRININ Citrinin can co-occur with OTA, is produced by both Penicillium and Aspergillus, and like OTA targets the kidney (Kitchen et al., 1977). Symptoms of pruritis, pyrexia and hemorrhagic syndrome in a dairy herd were attributed to citrinin (Griffiths and Done, 1991).
PATULIN
Patulin is produced by Penicillium, Aspergillus, and Byssochlamys and may be found in silage (Dutton et al., 1984; Hacking and Rosser, 1981). Patulin has been incriminated as a possible toxin in Europe and New Zealand (Lacey, 1991).
PR TOXIN Produced by Penicillium roquefortii, PR toxin has been found in silage (Hacking and Rosser, 1981) and was the suspected vector in a case study with symptoms of abortion and retained placenta (Still et al., 1972). Surveys of grass and corn silage in Europe have found P. roquefortii in up to 40% of samples (Auerbach, 2003).
Table 4. US FDA guidance for industry on fumonisin levels in human foods and animal feeds.1
| Total fumonisins (FB1+FB2+FB3) (ppm) | ||
| Human foods | ||
| Product | ||
| Degermed dry milled corn products (e.g., flaking grits, corn grits, corn meal, corn flour with fat content of < 2.25%, dry weight basis) | 2 | |
| Whole or partially degermed dry milled corn products (e.g., flaking grits, corn grits, corn meal, corn flour with fat content of ≥ 2.25 %, dry weight basis) | 4 | |
| Dry milled corn bran | 4 | |
| Cleaned corn intended for masa production | 4 | |
| Cleaned corn intended for popcorn | 3 | |
| Animal feeds | ||
| Corn and corn by-products intended for: | ||
| Equids and rabbits (no more than 20% of diet)2 | 5 | |
| Swine and catfish (no more than 50% of diet)2 | 20 | |
| Breeding ruminants, breeding poultry and breeding mink and including lactating dairy cattle and hens laying eggs for human consumption (no more than 50% of diet)2 | 30 | |
| Ruminants ≥3 months old being raised for slaughter and mink being raised for pelt production (no more than 50% of diet)b | 60 | |
| Poultry being raised for slaughter (no more than 50% of diet)2 | 100 | |
| All other species or classes of livestock and pet animals (no more than 50% of diet)2 | 10 | |
| 1 Federal Register, 2001. 2 Limits on ingredients are on a dry weight basis |
DICOUMAROL
Dicoumarol is produced from natural plant compounds when Penicillium or Aspergillus molds grow on sweet clover or sweet vernal grass. Dicoumarol interferes with the function of vitamin K, resulting in a hemorrhagic syndrome. Moldy sweet clover poisoning is discussed by Radostits et al. (1980).
ERGOT ALKALOIDS
One of the earliest recognized mycotoxicoses is ergotism caused by a group of ergot alkaloids. They are produced by several species of Claviceps, which infect the plant and produce toxins in fungal bodies called sclerotia or ergots. Ergotism primarily causes a nervous or gangrenous condition in animals. Symptoms are directly related to dietary concentrations and include reduced weight gains, reduced milk production, and agalactia (Robbins et al., 1986). Sclerotia concentrations above 0.3% are related to reproductive disorders. Fescue infected with Neotyphodium or Epichloe may contain toxic alkaloids associated with ‘fescue toxicity’ (CAST, 2003). Fescue is a major pasture grass in the US, growing widely throughout the lower midwest and upper south. Over half of the fescue is endophyte-infected, making this a serious problem for cattle and horse producers. Endophyte-free varieties are available, but they are not as hardy as infected varieties. Fescue infected with a nonpathogenic endophyte may be more field hardy and less toxic.
Are dairy products contaminated when dairy cattle consume mycotoxins?
Moy (1998) reviewed the international efforts to evaluate and reduce the human risks of mycotoxins. He stated that “human health problems caused by the consumption of most mycotoxins are complex and poorly understood”, but they may be responsible for a range of diseases. The majority of human health risk from mycotoxins is from consumption of contaminated grains and nuts. While many mycotoxins are common contaminants of feedstuffs and several mycotoxins have been shown to occur in the milk of dairy cattle, concentrations are extremely low because only a small fraction of the amount consumed by a cow is transferred to milk in the parent form or as a derivative. Aflatoxin is the only mycotoxin that has received regulatory action in the US as a possible contaminant in milk. This is because aflatoxin transfer from feed to milk is greater than for other mycotoxins. Also, aflatoxin is carcinogenic, highly toxic to humans, and because milk is a primary component of the diet of infants. The US FDA indicated that aflatoxin is the only mycotoxin that currently warrants regulation in milk (Wood and Trucksess, 1998).
AFLATOXIN
Milk aflatoxin residues are the result of transformation of the parent compound in the liver and its subsequent secretion into milk. Aflatoxin B1 results in milk residues of aflatoxin M1, while aflatoxin B2 results in milk residues of aflatoxin M2. Small amounts of other derivatives such as aflatoxin M4, Q1, and aflatoxicol can also be found in milk; however aflatoxin M1 is the primary residue (Wood, 1991). Van Egmond (1989) concluded that aflatoxin carryover from feed to milk is approximately 1-2%.
Frobish et al. (1986) found greater aflatoxin transfer to milk when the toxin was supplied by contaminated cottonseed meal than when it was supplied by contaminated corn. Percentage transfer of aflatoxin to milk was not affected by concentration in the feed or by milk production level of the cow. They concluded that concentration of aflatoxin M1 in milk was approximately equal to 1.51% of the concentration of aflatoxin B1 in the diet. Therefore a concentration of 33 ppb in the total diet would result in a 0.5 ppb concentration in milk (3.9 ppb in the milk dry matter, assuming 12.8% milk solids). Figure 1 shows the extent to which four toxin adsorbents added to the diet of dairy cows reduced aflatoxin M1 in milk (Diaz et al., 1999).
Regulatory pressures and a widespread awareness have helped minimize aflatoxin problems. Surveys of aflatoxin B1 concentrations in feedstuffs conducted during the 1980s resulted in lower levels than for surveys conducted in the 1970s (Van Egmond, 1989).
Figure 1. Effects of sequestering agents on milk aflatoxin residues (Diaz et al., 1999).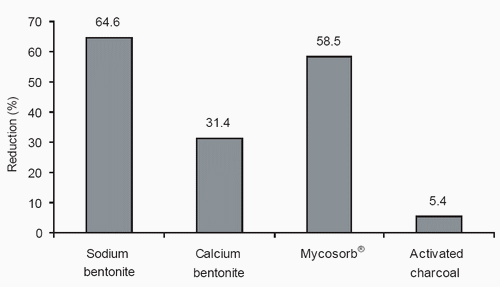
The United States General Accounting Office (GAO, 1991) concluded that industry, federal and state programs are effective in detecting and controlling aflatoxin and that it is doubtful that additional programs or limits would reduce the risk of aflatoxin in the food supply. The GAO specifically examined the state-administered program in the state of Georgia as a part of its report. In 1989, 13% of corn samples tested by the Georgia Department of Agriculture exceeded 20 ppb. On farms, 3.9% of tested milk exceeded limits while at the retail level only 0.4% of milk was in violation. Current surveillance programs in the US aimed at reducing food residues make it very unlikely that aflatoxin will be fed at high enough levels and for sufficient duration to have significant production or health effects on dairy herds in those regions that have an active program.
Dairy cattle feeds should contain less than 20 ppb aflatoxin to prevent milk residues above 0.5 ppb. Concentrations of aflatoxin should be conservatively low because of uncertainties in sampling and analysis, nonuniform distribution of aflatoxin, and potential for more than one source of aflatoxin in the diet.
DEOXYNIVALENOL
DON is changed to DOM-1 in the rumen with estimates of 24 hr degradation of about 50% (King et al., 1984). Deoxynivalenol and metabolites are rapidly excreted, primarily through urine (Côté et al., 1986; Prelusky et al., 1984; Prelusky et al., 1987). Prelusky et al. (1984) administered DON in an oral dose of 920 mg and found less than 4 ppb of free and conjugated DON in the milk. DON was excreted in milk primarily as DOM-1, but excretion rate is extremely low at 0.0001% of the dose. Côté et al. (1986) found no DON, but up to 30 ppb of DOM-1 in milk of cows fed DON at about 300 mg/day (66 ppm) for five days.
ZEARALENONE
Shreeve et al. (1979) fed dairy cows about 1 ppm zearalenone for 11 weeks without detecting a milk residue. Prelusky et al. (1990) administered up to 6 g of zearalenone per cow daily and found a total milk residue of up to 16 ppb, which represented about 0.01% of the dose. Hagler et al. (1980) administered 5 g zearalenone in ground feed to a lactating dairy cow that was milked twice daily with samples collected until 120 hr after dosing. Only trace levels of zearalenone were found in the milk obtained at 96, 108 and 120 hr after dosing and trace levels of zearalenol were also found in the milk at 108 and 120 hr after dosing. Mirocha et al. (1981) found that zearalenone and its metabolites reached levels above 1 ppm in milk representing about 0.7% of the zearalenone dosage, which was 25 ppm for eight days.
T-2 TOXIN
Residues of T-2 and its derivatives have been found in milk, but have a low transfer rate from feed to milk. After 72 hrs, an orally administered dose of T- 2 at 0.42 mg/kg of body weight (approximately 36 ppm) was almost completely excreted in the feces and urine (Yoshizawa et al., 1981; Yoshizawa et al., 1982). Milk residues, which reached a maximum of about 35 ppb, suggest that about 0.2% of T-2 and its metabolites are secreted in milk. In the lactating cow administered radioactive labeled T-2 toxin, three metabolites (3'-hydroxy-T-2 toxin, 3'-hydroxy-HT- 2 toxin and 3'-hydroxy-7-hydroxy-HT-2 toxin) accounted for 30-40% of the radioactivity in urine, 60-70% of radioactivity in milk and 50-60% of the radioactivity in blood plasma. Other metabolites included HT-2 toxin, neosolaniol and 4- deacetylneosolaniol. Other investigators (Robinson et al., 1979) have measured T-2 up to a peak of 160 ppb in milk on the fifth day after starting oral intubation with daily doses of 182 mg of T-2 toxin for 15 consecutive days (equivalent to about 9 ppm in the diet, assuming a daily consumption of 20 kg).
FUMONISIN
Fumonisin B1 carryover from feed to milk is thought to be negligible (Richard et al., 1996; Scott et al., 1994). Prelusky et al. (1996) reported studies where dairy cattle were administered fumonisin B1 either orally or intravenously. The oral dosages were approximately equal to dietary concentrations of 60 to 300 ppm. The intravenous dosages were stated to be similar to dietary concentrations of 125 to 500 ppm. No fumonisin B1 or its metabolites were detected in milk (detection limit of 0.5 ppb for fumonisin B1). Maragos and Richard (1994) analyzed 155 milk samples collected in Wisconsin during a period when feeds were reported to be severely affected by mold. Additionally, 10 samples were collected in Illinois. Feed samples associated with these milk samples were not collected and thus fumonisin B1 concentrations in feed were unknown. Only one of the 165 milk samples tested positive for fumonisin B1, which was determined to be 1.29 ppb. This suggests that fumonisin can occur in milk, but is likely to be at very low levels.
OCHRATOXIN
Goats were administered a single dose of radiolabeled ochratoxin A at 0.5 mg/kg (Nip and Chu, 1979). Cumulative excretion of radioactivity over seven days indicated that 53% was excreted in the feces, 38% in the urine and 6% in milk. Of the radioactivity in milk, only a small amount was in the form of ochratoxin A (OTA), representing 0.026% of the dosage administered. In a study with lactating cows where ochratoxin A was fed at 317 to 1,125 ppb for 11 weeks, neither ochratoxin A nor its metabolite ochratoxin α were detected in milk (Shreeve et al., 1979).
SUMMARY
Several other mycotoxins, or their derivatives, may be found in extremely small amounts in milk. Other than aflatoxin, they are not considered likely human health hazards in milk. It is thought that significant residues of these other mycotoxins occur in milk only when very high, nonclinical levels are administered to cows. Additionally the derivatives are generally less toxic than the parent compound. Aflatoxin is the only mycotoxin that has received regulatory action in the US as a possible contaminant in milk. Regulatory efforts have successfully reduced the risk of aflatoxin in the food supply in the US (GAO, 1991). Efforts to prevent aflatoxin formation, to divert contaminated ingredients away from dairy feeds usage, and to use feed additives that reduce aflatoxin absorption by the animal, have contributed to fewer milk contamination problems.
What are the safe levels of mycotoxins for dairy cattle?
Some of the same factors that make diagnosis difficult also contribute to the difficulty of establishing levels of safety. These include lack of research, sensitivity differences among animal species, imprecision in sampling and analysis, the large number of potential mycotoxins, and interactions with other mycotoxins and stress factors (Hamilton, 1984; Schaeffer and Hamilton, 1991). Mycotoxin effects are also moderated by factors such as sex, age, duration of exposure, and stresses of the environment and production. The known dietary factors that interact with mycotoxins include nutrients such as fat, protein, fiber, vitamins and minerals (Brucato et al., 1986; Coffey et al., 1989; Smith et al., 1971).
Naturally contaminated feeds are more toxic than feeds with the same level of a pure mycotoxin supplemented into the diet. Jones et al. (1982) demonstrated that productivity losses in commercial broiler operations can occur when aflatoxin concentrations are below those shown by controlled research to be of concern in laboratory situations. Aflatoxin produced from culture was more toxic to dairy cattle than pure aflatoxin added to diets (Applebaum et al., 1982). In swine, Foster et al. (1986) demonstrated that a diet containing pure added DON was less toxic than diets with similar concentrations of DON, which were supplied from naturally contaminated feeds. Smith and MacDonald (1991) have suggested that fusaric acid, produced by many species of Fusarium, occurs along with DON to produce more severe symptoms. Lillehoj and Ceigler (1975) give an example where penicillic acid and citrinin were innocuous in laboratory animals when administered alone but were 100% lethal when given in combination. These studies strongly suggest the presence of other unidentified mycotoxins in naturally contaminated feeds. It is well documented that several mycotoxins may be found in the same feed (Hagler et al., 1984). Abbas et al. (1989) demonstrated that Fusarium species isolated from Minnesota corn produced multiple mycotoxins. Because animals are fed a blend of feedstuffs and because molds produce an array of mycotoxins, many mycotoxin interactions are possible.
Interactions with other stress factors make recommendations difficult. Animals under environmental or production stress may show more pronounced symptoms. It is clearly shown that there is a temperature interaction with fescue toxicity such that more pronounced symptoms are expressed during heat stress (Bacon, 1995). Fumonisin at 100 ppm has been shown to reduce milk production in dairy cattle (Diaz et al., 2000), and in a separate study to not significantly affect average daily gain in beef cattle fed 148 ppm (Osweiler et al., 1993). While this contrast may reflect a difference in the duration of feeding, number of animals studied, etc., it may also suggest differences due to greater stress in early lactation dairy cattle as compared with young growing beef cattle.
Because of partial degradation in the rumen, mycotoxins are less toxic to cattle than to most other animals. However mycotoxins are not completely degraded and some of the degradation products remain toxic (Kiessling et al., 1984). Extent of ruminal degradation appears to be variable. It is speculated that feeding situations resulting in a faster rate of ruminal feed passage or a low population of protozoa in the rumen may reduce mycotoxin degradation in the rumen. Ruminal degradation of mycotoxins appears to be more dependent on protozoal than bacterial activity (Kiessling et al., 1984).
Which feed mycotoxin source is the greatest problem - grains or silages?
Almost any type of feed can be contaminated with mycotoxins. Table 5 compares the incidence and concentrations of mycotoxins in corn grain and corn silage over a nine year period. Perhaps the worst case scenario for a dairy producer may be to have the onfarm stored feeds contaminated. Therefore, the season’s supply of feed is contaminated and not just the current load that is used over a short time period. This is because toxicity is a function not only of amount but also duration of feeding. When the onfarm stored feed is contaminated, the dairy producer faces a difficult decision. The contaminated feed could be fed as normal, diluted with purchased feed or not used. Increased costs may be incurred from additional feed purchases or reduced income may result from a loss in animal performance. The contaminated feed may be either grain or forage; however, dairy producers are more likely to store forage than grain. Therefore, it is important to discuss mycotoxin contamination of forages.
Many different mycotoxins have been found to occur in forages either in the field, or in storage as hay or silage (Lacey, 1991). Some mycotoxicoses in cattle resulting from contaminated forages have been reviewed (Lacey, 1991; Gotlieb, 1997; Seglar, 1997; Whitlow, 1993; Whitlow and Hagler, 1997). It is unclear how much of the mycotoxin contamination of forages occurs prior to harvest. Fresh feed can be contaminated with mycotoxins at harvest; however, mold can grow in harvested forages. The limiting factor for mold growth in hay is low moisture content. When hay is stored too wet, mold is likely to grow, produce heat and cause heat damage. The limiting factors for mold growth in silage are a low pH and high moisture, which limit air infiltration. If silage is stored too dry or insufficiently packed and covered, infiltration of air allows for microbial activity which depletes silage acids, allowing pH to rise and molds to grow. Some of the Penicillium molds grow at a low pH (Auerbach, 2003). Silage on the feeding face must be removed at a rate that prevents air infiltration into the silage mass resulting in conditions that support mold growth. Practical recommendations are to feed 6 to 12 inches daily from the feeding face to prevent mold.
Table 5. Occurrence of five mycotoxins in corn silage and corn grain in samples submitted for analysis by producers in North Carolina over a nine-year period1.
| Aflatoxin(>10 ppb) | Deoxynivalenol(>50 ppb) | Zearalenone(>70 ppb) | T-2 toxin(>50 ppb) | Fumonisin(>1 ppm) | ||||||||||
| Corn silage | ||||||||||||||
| n | Positive (%)2 | X ± sd | n | Positive (%) | X ± sd | n | Positive (%) | X ± sd | n | Positive (%) | X ± sd | n | Positive (%) | X ± sd |
| 461 | 8 | 28 ± 19 | 778 | 66 | 1991 ± 2878 | 487 | 30 | 525 ± 799 | 717 | 7 | 569 ± 830 | 63 | 37 | N/A |
| Corn grain | ||||||||||||||
| 231 | 9 | 170 ± 606 | 362 | 70 | 1504 ± 2550 | 216 | 11 | 206 ± 175 | 353 | 6 | 569 ± 690 | 37 | 60 | N/A |
| 1Whitlow et al., 1998 n = number of samples 2Percentage of samples positive above given concentrations |
It appears that Aspergillus flavus does not grow well in hay or silage, however, aflatoxin concentrations up to 5 ppm have been reported (Kalac and Woolford, 1982). We have detected low levels of aflatoxin (<100 ppb) in corn silage and alfalfa. Table 5 shows that the frequency of aflatoxin in corn silage is not different from the frequency of aflatoxin in corn grain, but the concentrations are lower. The frequency and concentrations of some Fusarium-produced mycotoxins are also compared in Table 5. There is a trend toward a higher frequency of ZEN in corn silage than in corn grain.
Aspergillus fumigatus is thought to be a fairly common mold in both hay (Shadmi et al., 1974) and silage (Cole et al., 1977) and may be a vector in the development of mycoses in dairy cattle. Silage was found to contain fumigaclavine A and C and several fumitremorgens (Cole et al., 1977). Animal symptoms included generalized deterioration typical of protein deficiency, malnutrition, diarrhea, irritability, abnormal behavior and occasionally death. The hay was fed to goats and rats and resulted in retarded growth and histopathological changes in the livers and kidneys.
Surveys of grass and corn silage in Europe have found an occurrence of P. roquefortii in as many as 40% of samples (Auerbach, 2003). PR toxin, produced by Penicillium roquefortii, has been found in silage (Hacking and Rosser, 1981) and was the suspected vector in a case study with symptoms of abortion and retained placenta (Still et al., 1972). Vesely et al. (1981) reported that in cows fed corn silage containing PR-toxin there was loss of appetite, gut inflamation, rumen stasis and abortion. Moldy alfalfa hay containing Aspergillus ochraceus was implicated as producing OTA associated with abortions in cattle (Still et al., 1971). OTA in moldy forage has also been implicated in cattle deaths (Vough and Glick, 1993).
The most important pasture-induced toxicosis in the US is tall fescue toxicosis caused by endophytic alkaloids (Bacon, 1995). Other forage toxicoses of fungal origin include ergotism, perennial ryegrass staggers, slobbers syndrome (a hemorrhagic disease that is associated with dicoumarol produced in fungal infected sweet clover and sweet vernal grass), and syndromes of unthriftiness and impaired reproduction associated with Fusarium (Cheeke, 1995).
How can mycotoxin effects be reduced?
Pre-harvest control has involved agronomic practices that minimize mycotoxin accumulation in the field. These include proper irrigation, pesticide application, resistant or adapted hybrids, proper tillage and fertilization. Unfortunately, breeding for mycotoxinresistant hybrids has been only partially successful. Munkvold et al. (1999) have shown that compared with nontransgenic corn, Bacillus thurengiensis (Bt)- transgenic corn had less corn borer damage, less F. verticillioides infection, and lower fumonisin contamination. Fungicides have shown little efficacy in controlling pre-harvest aflatoxin contamination in corn (Duncan et al., 1994).
Post-harvest approaches for management of mycotoxin contamination include mycotoxin analysis of feedstuffs and diversion of contaminated lots; ammoniation of corn and cottonseed to destroy aflatoxin; dilution; and storage technology (Trail et al., 1995). Mycotoxin-contaminated grains can be used for ethanol production, and in some cases mycotoxin-contaminated grains can be diluted with clean feeds (Desjardins et al., 1993). The FDA does not allow dilution of aflatoxin-contaminated feeds, which is considered adulteration. The best strategy for post-harvest control of mycotoxins is proper storage and handling of feed grains.
Sampling and testing feeds is a part of a control program. The accurate determination of mycotoxin concentrations in grain and feeds depends on a number of factors. First, a statistically valid sample must be drawn from the lot (Whittaker et al., 1991). Because mycotoxins are not evenly distributed in grains and other feedstuffs, most of the error in a single analysis is due to sampling – as much as 90% of the error is associated with the taking of the initial sample. Proper collection and handling of representative feed samples is essential. Once collected, samples should be handled properly to prevent further mold growth. Wet samples may be frozen or dried before shipment and transit time should be minimized. The sample must then be finely ground and subsampled for analysis; this step is the second largest source of error in an analysis. Finally, the subsample is extracted, extract purified using one of several techniques, and then the toxin is measured. Toxin determination may be by thin-layer chromatography plates (TLC), highperformance liquid chromatography (HPLC), gas-liquid chromatography (GLC), enzyme-linked immunosorbent assays (ELISA), spectrophotometrically, or by other techniques. Blacklighting for bright-greenish-yellow fluorescence is often used as a screening technique for aflatoxin, but it is very inaccurate; newer and better methods should be used. As far as we are aware, blacklighting is completely inappropriate for other mycotoxins. Mold spore counts may not be very useful and are only a gross indication of the potential for toxicity, but mold identification can be useful to suggest which mycotoxins may be present. Scott (1990) states that screening methods are needed for the Fusariumproduced mycotoxins and that one approach is to test first for DON, DAS, T-2 toxin and nivalenol, because other Fusarium mycotoxins seldom occur without one of these four also present. Feeds could then be tested for other mycotoxins if necessary.
Generally, laboratories provide analysis for only a limited number of mycotoxins, perhaps including aflatoxin, ochratoxin, deoxynivalenol, zearalenone, fumonisin, and T-2 toxin. Minimum detection levels may be limiting because they are often directed at finding high levels that cause serious animal disease, rather than low levels which are associated with production losses, impaired immunity and significant economic losses. However, analytical techniques for mycotoxins are improving, costs are decreasing and several commercial laboratories are available which provide screens for an array of mycotoxins. The Federal Grain Inspection Service (USDA-GIPSA) provides on the internet a list of approved mycotoxin tests for grains and provides excellent background materials for the feed industry (internet address http:/ /www.usda.gov/gipsa/pubs/mycobook.pdf).
Laboratory methods can be found in Official Methods of Analysis of AOAC International (Horwitz, 2000). The potential for effective treatments has improved. Certain feed additives can reduce mycotoxin exposure of animals and thus minimize their negative effects. Some additives may be beneficial in reducing mycotoxin formation because they are effective in reducing mold growth. Ammonia, propionic acid, microbial and enzymatic silage additives have all shown some effectiveness as mold inhibitors. Additives to enhance fermentation can be added at ensiling. Mold growth inhibitors may be helpful as a surface treatment when capping off the silo or daily after silage feed-out to reduce molding of the exposed silage feeding face. If unacceptably high levels of mycotoxins occur, dilution or removal of the contaminated feed is preferable; however, it is usually impossible to replace all of a major forage ingredient. While dilution is sometimes a viable practice to reduce mycotoxin exposure, reduced feeding of silage could result in such a slow feedout, that mycotoxin problems within the silage increase. Ammoniation of grains can destroy some mycotoxins, but there is no practical method to detoxify affected forages already in storage. A microbial detoxification method (Binder et al., 2000) has been identified where a species of rumen bacterium (BBSH 797) was isolated which has the ability to biotransform DON, rendering it non-toxic. Field studies have suggested that the microbial product used as a feed additive can protect growing pigs from the effects of DON. Galvano et al. (2001) has reviewed dietary strategies to counteract mycotoxins. Increasing nutrients such as protein, energy and antioxidant nutrients may be advisable (Brucato et al., 1986; Coffey et al., 1989; Smith et al., 1971).
Sequestering agents such as clays (bentonites) added to contaminated diets fed to rats, poultry, swine and cattle have helped reduce the effects of mycotoxins (Diaz et al., 1997; Galey et al., 1987; Harvey, 1988; Kubena et al., 1993; Lindemann and Blodgett, 1991; Scheideler, 1993; and Smith, 1980; 1984). In most cases, clay has been added to the diet at about 1%. Activated carbon at 1% of the diet effectively reduced aflatoxin in milk (Galvano et al., 1996). Activated carbon fed at 0.1% of the diet did not reduce aflatoxin levels in milk (Diaz et al., 1999). A glucomannan (Mycosorb®) fed at 0.05% of diet dry matter or bentonites at 1% of diet dry matter were similarly effective in reducing aflatoxin concentrations in milk (Diaz et al., 1999) (Figure 1). The low inclusion rate of the glucomannan in comparison to clay-type absorbants may be an important difference. A recent review of mycotoxin binders provides more details, but only limited comparison data are available (Huwig et al., 2001).
What policy changes and research are needed?
The CAST (2003) publication listed a number of important needs for research and public policy. We have summarized a few of those. It is obvious that current information and understanding of mycotoxins is inadequate; and for animal agriculture there is a critical need for better methods of diagnosis, prevention and treatment.
1. Ensure a safe food supply.
2. Develop uniform worldwide standards and regulations for mycotoxin contamination.
3. Improve mycotoxin analyses to be more definitive, quicker, simpler and cheaper.
4. Develop better diagnostics to include biomarkers to detect animal exposure to mycotoxins.
5. Assess mycotoxins as virulence factors.
6. Investigate the immunosuppressing effects of mycotoxins.
7. Investigate the toxicological interactions of toxins with the host.
8. Investigate possible genetic differences in sensitivity to mycotoxins and the genetics of mycotoxin production by fungi.
9. Assess interactions among mycotoxins and with drugs, diet and nutrition.
10. Develop a better understanding of factors affecting mycotoxin formation in the field and in storage.
11. Improve understanding of the ecology and epidemiology of mycotoxin-producing fungi.
12. Develop sound agronomic management practices to reduce mycotoxin contamination.
13. Develop host-plant resistance to toxigenic fungi and to mycotoxin occurrence.
14. Develop better models to predict the potential for mycotoxin formation.
15. Develop better sampling protocols.
References
Abbas, H.K., C.J. Mirocha, R.A. Meronuck, J.D. Pokorny, S.L. and T. Kommedahl. 1988. Mycotoxins and Fusarium spp. associated with infected ears of corn in Minnesota. Appl. Env. Micro. 54:1930-1933.
Abbas, H.K., C.J. Mirocha, T. Kommedahl, R.F. Vesonder and P. Golinski. 1989. Production of trichothecenes and non-trichothecene mycotoxins by Fusarium species isolated from maize in Minnesota. Mycopathologia. 108:55-58.
Anonymous, 1995. USDA. APHIS. Mycotoxin Levels in the 1995 Midwest Preharvest Corn Crop. Veterinary Services Factsheet N195.1295. The National Veterinary Services Laboratory, Ames, Iowa.
Applebaum, R.S., R.E. Brackett, D.W. Wiseman, and E.L. Marth. 1982. Responses of dairy cows to dietary aflatoxin: feed intake and yield, toxin content, and quality of milk of cows treated with pure and impure aflatoxin. J. Dairy Sci. 65:1503-1508.
Ashworth, L.J. Jr., J.L. McMeans and C.M. Brown. 1969. Infection of cotton by Aspergillus flavus and epidemiology of the disease. J. Stored Prod. Res. 5:193-202.
Auerbach, H. 2003. Mould growth and mycotoxin contamination of silages: sources, types and solutions. In: Biotechnology in the Feed Industry. (T. P. Lyons and K. A. Jacques, eds. Nottingham University Press, UK, pp. 247-265.
Bacon, C.W. 1995. Toxic endophyte-infected tall fescue and range grasses: Historic perspectives. J. Anim. Sci. 73:861-864.
Bauer, J., A. Gareis, A. Gott, and B. Gedek. 1989. Isolation of a mycotoxin (gliotoxin) from a bovine udder infected with Aspergillus fumigatus. J. Med. Vet. Mycol. 27:45–50.
Binder, E. M., D. Heidler, G. Schatzmayr, N. Thimm, E. Fuchs, M. Schuh, R. Krska, and J. Binder. 2000. Microbial detoxification of mycotoxins in animal feed. In: Mycotoxins and Phycotoxins in Perspective at the Turn of the Millennium, Proceedings of the 10th International IUPAC Symposium on Mycotoxins and Phycotoxins, Guarujá, Brazil, ICC Publications, Vienna, Austria, pp 271-278.
Boyacioglu, D., N.S. Hettiarachchy and R.W. Stack. 1992. Effect of three systemic fungicides on deoxynivalenol (vomitoxin) production by Fusarium graminearum in wheat. Can. J. Plant Sci. 72:93-101.
Brucato, M., S.F. Sundlof, J.U. Bell and G.T. Edds. 1986. Aflatoxin B1 toxicosis in dairy calves pretreated with selenium-vitamin E. Am. J. Vet. Res. 47:179-183.
CAST. (Council for Agricultural Science and Technology). 1989. In: Mycotoxins: Economics and Health Risks. Task Force Report No. 116. Ames, IA.
CAST. (Council for Agricultural Science and Technology). 2003. In: Mycotoxins in Plant Animal and Human Systems. Task Force Report No.139. Ames, IA.
Cawood, M.E.,W.C.A. Gelderblom, R. Vlegaar, Y. Behrend, P.G. Thiel and W.F.O. Marasas. 1991. Isolation of the fumonisin mycotoxins: a quantitative approach. J. Agric. Food Chem. 39:1958-1962.
Charmley, E., H.L. Trenholm, B.K. Thompson, D. Vudathala, J.W.G. Nicholson, D.B. Prelusky and L.L. Charmley. 1993. Influence of level of deoxynivalenol in the diet of dairy cows on feed intake, milk production and its composition. J. Dairy Sci. 76:3580-3587.
Cheeke, P.R. 1995. Endogenous toxins and mycotoxins in forage grasses and their effects on livestock. J. Anim. Sci. 73:909-918.
Cheeke, P.R. and L.R. Shull. 1985. Natural Toxicants in Feeds and Poisonous Plants. The AVI Publishing Company, Westport, CT.
Christensen, C.M., C.J. Mirocha and R.A. Meronuck. 1977. Molds Mycotoxins and Mycotoxicoses. Agricultural Experiment Station Miscellaneous Report 142. University of Minnesota, St. Paul, MN.
Christensen, C.M., C.J. Mirocha and R.A. Meronuck 1988. Molds and Mycotoxins in Feeds. Minn. Ext. Serv. Bull. AG-FO-3538. Univ. MN, St. Paul.
Chu, F.S. and G. Y. Li. 1994. Simultaneous occurrence of fumonisin B1 and other mycotoxins in moldy corn collected from the People’s Republic of China in regions with high incidences of esophageal cancer. Appl. Environ. Microbiol. 60:847-852.
Coffey, M.T., W.M . Hagler, Jr. and J.M. Cullen. 1989. Influence of dietary protein, fat, or amino acids on the response of weanling swine to aflatoxin B1. J. Anim. Sci. 67:465-469.
Cole, R.J. and R.H. Cox. 1981. Handbook of Toxic Fungal Metabolites. Academic Press, New York.
Cole, R.J., J.W. Kirksey, J.W. Dorner, D.M. Wilson, J.C. Johnson, Jr., A.N. Johnson, D.M. Bedell, J.P. Springer, K.K. Chexal, J.C. Clardy and R.H. Cox. 1977. Mycotoxins produced by Aspergillus fumigatus species isolated from moldy silage. J. Agric. Food Chem. 25:826-830.
Cook, M.E., and W. Wu. 1991. Basic aspects of mycotoxins. In: 4-State Applied Nutrition Conference, Univ. of Wisconsin, Madison, pp 97-104
Coppock, R.W., M.S. Mostrom, C.G. Sparling, B. Jacobsen and S.C. Ross. 1990. Apparent zearalenone intoxication in a dairy herd from feeding spoiled acid-treated corn. Vet. Hum. Toxicol. 32:246-248.
Côté, L.M., A.M. Dahlem, T. Yoshizama and W.B. Buck. 1986. Excretion of deoxynivalenol and its metabolite in milk, urine and feces of lactating dairy cows. J. Dairy Sci. 69:2416.
Cotty, P.J., P. Bayman, D.S. Egel and D.S. Elias. 1994. Agriculture, aflatoxins and Aspergillus. In: The Genus Aspergillus. (K.A. Powell, A. Fenwick and J.F. Peberdy, eds) Plenum Press. New York, pp. 1-27
Coulumbe, R.A. 1993. Symposium: biological action of mycotoxins. J. Dairy Sci. 76:880-891.
Davis, N.D. and U.L. Deiner. 1983. Some characteristics of toxigenic and nontoxigenic isolates of Aspergillus flavus and Aspergillus parasiticus. In: Aflatoxin and Aspergillus flavus in Corn. (U.L Diener, R.L. Asquith and J. W. Dickens, eds.) Southern Coop Series Bull 279, Craftmaster, Opelika, AL
DeCostanzo, L. Johnston, L. Felice and M. Murphy. 1995. Effects of molds on nutrient content of feeds reviewed. Feedstuffs 67:17-18,20,52-54.
Deiner, U.L., R.J. Cole, T.H Sanders, G.A. Payne, L.S. Lee and M.A. Klich. 1987. Epidemiology of aflatoxin formation by Aspergillus flavus. Ann. Rev. Phytopathology 25:240-270.
Desjardins, A.E. and T.M. Hohn. 1997. Mycotoxins in plant pathogenesis. Molec. Plant-Microbe Interact. 10:147–152.
Desjardins, A.E., T.M. Hohn and S.P. McCormick. 1993. Trichothecene biosynthesis in Fusarium species: chemistry, genetics and significance. Microbiol. Reviews 57:594-604.
Desjardins, A.E., Munkvold, G.P., Plattner, R.D., Proctor, R.H. 2002. FUM1—A gene required for fumonisin biosynthesis but not for maize ear rot and ear infection by Gibberella moniliformis in field tests. Molecular Plant-Microbe Interactions 15:1157-1164.
Diaz, D.E., J.T. Blackwelder, W.M. Hagler, Jr., B.A. Hopkins, F.T. Jones, K.L. Anderson and L.W. Whitlow. 1997. The potential of dietary clay products to reduce aflatoxin transmission to milk of dairy cows. J. Dairy Sci. 80(abstr.):261.
Diaz, D.E., W.M. Hagler, Jr., B.A. Hopkins, J.A. Eve and L.W. Whitlow. 1999. The potential for dietary sequestering agents to reduce the transmission of dietary aflatoxin to milk of dairy cows and to bind aflatoxin in vitro. J. Dairy Sci. 82(abstr.):838.
Diaz, D.E., B.A. Hopkins, L.M. Leonard, W.M. Hagler, Jr., and L.W. Whitlow. 2000. Effect of fumonisin on lactating dairy cattle. J. Dairy Sci. 83(abstr.):1171.
DiCostanzo, A.L. Johnston, H. Windels and M. Murphy. 1995. A review of the effects of molds and mycotoxins in ruminants. Professional Animal Scientist 12:138-150.
Diekman, D.A. and M.L. Green. 1992. Mycotoxins and reproduction in domestic livestock. J. Anim. Sci. 70:1615-1627.
Duncan, H.E., A.R. Ayers, G.A. Payne, and W.M. Hagler, Jr. 1994. Lack of fungicidal control of Aspergillus flavus in field corn. In: Biodeterioration Research 4. (G.C. Llewellyn, W.V. Dashek, and C.E. O’Rear, eds.). Plenum Press, New York, pp. 161-175
Dutton, M.F., K. Westlake, and M.S. Anderson. 1984. The interaction between additives, yeasts and patulin production in grass silage. Mycopathologia 87:29-33.
Eichner, R.D., U. Tiwari-Palni, P. Waring, and A. Mullbacher. 1988. Detection of the immunomodulating agent gliotoxin in experimental aspergillosis. In: Proceedings of the Tenth Congress of International Society of Human and Animal Mycology, (J. M. Torres-Rodriguez ed.). Prous Scientific, Barcelona. pp. 133–137.
Foster, B.C., H.L. Trenholm, D.W. Friend, B.K. Thompson and K.E. Hartin. 1986. Evaluation of different sources of deoxynivalenol (vomitoxin) fed to swine. Can. J. Anim. Sci. 66:1149-1154.
Frobish, R.A., B.D. Bradley. D.D. Wagner, P.E. Long-Bradley and H. Hairston. 1986. Aflatoxin residues in milk of dairy cows after ingestion of naturally contaminated grain. J. Food Prot. 49:781-785.
GAO. 1991. Food safety and quality. Existing detection and control programs minimize aflatoxin. Report RCED-91-109.
Galey, F.D., R.J. Lambert, M. Busse and W.B. Buck. 1987. Therapeutic efficacy of superactive charcoal in rats exposed to oral lethal doses of T-2 toxin. Toxicon. 25:493-499.
Galvano, F., A. Pietri, T. Bertuzzi, G. Fusconi, M. Galvano, A. Piva, and G. Piva. 1996. Reduction of carryover of aflatoxin from cow feed to milk by addition of activated carbons. J. Food. Prot. 59:551-554.
Galvano, F., A. Piva, A. Ritieni, and G. Galvano. 2001. Dietary strategies to counteract the effects of mycotoxins: A review. J. Food Prot. 64:120–131.
Gareis, M. and J.Ceynowa. 1994. Influence of the fungicide Matador (tebuconazole/triadimenol) on mycotoxin production by Fusarium culmorum. Lebensmittel-Untersuchung-Forsch. 198:244-248.
Garrett, W.N., H. Heitman, Jr. and A.N. Booth. 1968. Aflatoxin toxicity in beef cattle. Proc. Soc. Exp. Biol. Med. 127:188-190.
Gelderblom, W.C.A., K. Jaskiewicz, W.F.O. Marasas, P.G. Thiel, R.M. Horak, R. Vleggaar and N.P.J. Kriek. 1988. Fumonisins: novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 54:1806-1811.
Gelderblom, W.C.A., N.P.J. Kreik, W.F.O. Marasas, and P.G. Thiel. 1991. Toxicity and carcinogenicity of the Fusarium moniliforme metabolite, fumonisin B1, in rats. Carcinogenesis 12:1247-1251.
Gentry, P.A., M.L. Ross and P.K-C Chan. 1984. Effect of T-2 toxin on bovine hematological and serum enzyme parameters. Vet. Hum. Toxicol. 26:24-24.
Gotlieb, A. 1997. Causes of mycotoxins in silages. In: Silage: Field to Feedbunk, NRAES-99, Northeast Regional Agricultural Engineering Service, Ithaca, NY, pp. 213-221.
Griffiths, I.B. and S.H. Done. 1991. Citrinin as a possible cause of pruritis, pyrexia, and haemorrhagic syndrome in cattle. Vet. Rec. 113-117.
Guthrie, L.D. 1979. Effects of aflatoxin in corn on production and reproduction in dairy cattle. J. Dairy Sci. 62 (abstr.):134.
Hacking, A. and W.R. Rosser. 1981. Patulin production by Paecilomyces species isolated from silage in the United Kingdom. J. Sci. Food. Agric. 32:620-623.
Hagler, Jr., W.M., C.J. Mirocha, S.V. Pathre, and J.C. Behrens, 1979. Identification of the naturally occurring isomer of zearalenol produced by Fusarium roseum ‘Gibbosum’ on rice. Appl. Environ. Microbiol. 37:849-853.
Hagler, Jr., W.M., G. Dankó, L. Horváth, M. Palyusik and C.J. Mirocha. 1980. Transmission of zearalenone and its metabolite into ruminant milk. Acta Vet. Hung. 28:209.
Hagler, Jr., W.M., K. Tyczkowska and P.B. Hamilton. 1984. Simultaneous occurrence of deoxynivalenol, zearalenone and aflatoxin in 1982 scabby wheat from the midwestern United States. Appl. Environ. Microbiol. 47:151-154.
Hagler, Jr., W.M., F.T. Jones, and D.T. Bowman. 1989. Mycotoxins in North Carolina 1985 crop soybeans. I. Zearalenone and deoxynivalenol in soybeans and soybean meal. In: Biodeterioration Research 2. (C.E.O’Rear and G.C. Llewellyn, eds.). Plenum Press, New York, pp. 337-349.
Hamilton, P.B. 1978. Fallacies in our understanding of mycotoxins. J. Food Prot. 41:404-408.
Hamilton, P.B. 1984. Determining safe levels of mycotoxins. J. Food Prot. 47:570-575.
Harris, L.J., A.E. Desjardins, R.D. Plattner, P. Nicholson, G. Butler, J.C. Young, G. Weston, R.H. Proctor, and T.H. Hohn. 1999. Possible role of trichothecene mycotoxins in virulence of Fusarium graminearum on maize. Plant Dis 83:954–960.
Harrison, L.R., B.M. Colvin, J.T. Greene, L.E. Newman, and R.J. Cole. 1990. Pulmonary edema and hydrothorax in swine produced by fumonisin B1, a toxic metabolite of Fusarium moniliforme. J Vet. Diagn. Invest. 2:217-221.
Harvey, R.B., L.F. Kubena, T.D. Phillips, W.E. Huff, and D.E. Corrier. 1988. Approaches to the prevention of aflatoxicosis. In: Proc. Maryland Nutr. Conf., University of Maryland, College Park. pp. 102-107.
Hawksworth, D.L. 1991. The fungal dimension of biodiversity:Magnitude, significance, and conservation. Mycol. Res. 95:641– 655.
Hesseltine, C.W. 1986. Resumé and future needs in the diagnosis of mycotoxins. In: Diagnosis of Mycotoxicoses. (J.L. Richard and J.R. Thurston, eds.). Martinus Nijhoff Publishers, Dordrecht, The Netherlands, pp. 381-385.
Horwitz, W. (ed.). 2000. Official Methods of Analysis of AOAC International. 17th ed. Association of Official Analytic Chemists International, Gaithersburg, Maryland.
Huwig, A., S. Freimund, O. Kappeli, and H. Dutler. 2001. Mycotoxin detoxication of animal feed by different adsorbents. Toxicol. Lett. 122:179–188.
Hsu, I.C., C.B. Smalley, F.M. Strong and W.E. Ribelin. 1972. Identification of T-2 toxin in moldy corn associated with a lethal toxicosis in dairy cattle. Appl. Microbiol. 24:684-690.
Ingalls, J.R. 1996. Influence of deoxynivalenol on feed consumption by dairy cows. Anim. Feed Sci. Tech. 60:297-300.
Joffe, A.Z. 1986. Fusarium Species: Their Biology and Toxicology. John Wiley and Sons, Inc., New York
Jones, F.T., W.M. Hagler, and P.B. Hamilton. 1982. Association of low levels of aflatoxin in feed with productivity losses in commercial broiler operations. Poultry Sci. 61:861-868.
Kalac, P. and M.K. Woolford. 1982. A review of some aspects of possible associations between the feeding of silage and animal health. Br. Vet. J. 138:305-320.
Kallela, K. and L. Vasenius. 1982. The effects of rumen fluid on the content of zearalenone in animal fodder. Nord. Vet. Med. 34:336-339.
Kallela, K. and E. Ettala. 1984. The oestrogenic Fusarium toxin (zearalenone) in hay as a cause of early abortions in the cow. Nord. Vet. Med. 36:305-309.
Kegl, T. and A. Vanyi. 1991. T-2 fusariotoxicosis in a cattle stock. Magyar Allatorvosok Lapja 46:467-471.
Khamis, Y., H.A. Hammad and N.A. Hemeida. 1986. Mycotoxicosis with oestrogenic effect in cattle. Zuchthyg. 21:233-236.
Kiessling, K.H., H. Patterson, K. Sandholm, and M. Olsen. 1984. Metabolism of aflatoxin, ochratoxin, zearalenone, and three trichothecenes by intact rumen fluid, rumen protozoa and rumen bacteria. Appl. Environ. Microbiol. 47:1070-1073.
King, R.R., R.E. McQueen, D. Levesque and R. Greenhalgh. 1984. Transformation of deoxynivalenol (vomitoxin) by rumen microorganisms. J. Agric. Food Chem. 32:1181.
Kitchen, D.N., W.W. Carlton and J. Tuite. 1977. Ochratoxin A and citrinin induced nephrotoxicosis in beagle dogs. I. Clinical and clinicopathological features. Vet. Pathol. 14:154-172.
Kosuri, N.R., M.D. Grave, S.G. Yates, W.H. Tallent, J.J. Ellis, I.A. Wolff and R.E. Nichols. 1970. Response of cattle to mycotoxins of Fusarium tricinctum isolated from corn and fescue. J. Am. Vet. Med. Assoc. 157:938-940.
Kriek, N.P.J., T.S. Kellerman, and W.F.O. Marasas. 1981. A comparitive study of the toxicity of Fusarium verticilloides (F. moniliforme) to horses, primates, pigs, sheep, and rats. Onderspoort J. Vet. Res. 48:129-131.
Krogh, P., F. Elling, C. Friis, B. Hald, A.E. Larsen, E.B. Lillehoj, A. Madsen, H.P. Mortensen, F. Rasmussen and U. Ravnskov. 1979. Porcine nephropathy induced by long-term ingestion of ochratoxin A. Vet. Pathol. 16:466-475.
Kubena, L.F., R.B. Harvey, W.E. Huff, M.H. Elissalde, A.G. Yersin, T.D. Phillips and G.E. Rottinghaus. 1993. Efficacy of a hydrated sodium calcium aluminosilicate to reduce the toxicity of aflatoxin and diacetoxyscirpenol. Poultry Sci. 72:51-59.
Kurtzman, C.P., B.W. Horn and C.W. Hesseltine. 1987. Aspergillus nomius, a new aflatoxinproducing species related to Aspergillus flavus and Aspergillus tamarii. Antonie van Leeuwenhoek 53:147-158.
Lacey, J. 1991. Natural occurrence of mycotoxins in growing and conserved forage crops. In: Mycotoxins and Animal Foods, (J. E. Smith and R. E. Henderson, eds.). CRC Press, Boca Raton, pp. 363-397.
Lillehoj, E.B., and A. Ciegler. 1975. Mycotoxin synergism. In: Microbiology, (D. Schlessinger, ed.) American Society of Microbiology, Washington, DC, pp. 344-358.
Lindemann, M.D. and D.J. Blodgett. 1991. Various clays provide alternative for dealing with aflatoxin. Feedstuffs 63:15-29.
Mann, D.D., G.M. Buening, B. Hook and G.D. Osweiler. 1983. Effects of T-2 mycotoxin on bovine serum proteins. J. Am. Vet. Med. Assoc. 44:1757-1759.
Maragos, C.M. and J.L. Richard. 1994. Quantitation and stability of fumonisins B1 and B2 in milk. JAOAC 77:1162-1167.
Marasas, W.F.O., P.E. Nelson and T.A. Toussoun 1984. Toxigenic Fusarium species, Identity and Mycotoxicology. Penn State Univ. Press, University Park.
Marasas, W.F.O., T.S. Kellerman, W.C.A. Gelderblom, J.A.W. Coetzer, P.G. Thiel and J.J. Van Der Lugt. 1988. Leucoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J. Vet. Res. 55:197-203.
Marsh, S.F. and G.A. Payne. 1984. Preharvest infection of corn silks and kernels by Aspergillus flavus. Phytopathology 74:1284-1289.
Masri, M.S., V.C. Garcia and J.R. Page. 1969. The aflatoxin M1 content of milk from cows fed known amounts of aflatoxin. Vet. Rec. 84:146-147.
Mirocha, C.J., J. Harrison, A.A. Nichols and M. McClintock. 1968. Detection of fungal estrogen (F-2) in hay associated with infertility in dairy cattle. Appl. Microbiol. 16:797-798.
Mirocha, C.J., B. Schauerhamer and S.V. Pathre. 1974. Isolation, detection and quantitation of zearalenone in maize and barley. J. Assoc. Off. Anal. Chem. 57:1104-1110.
Mirocha, C.J., S.V. Pathre and C.M. Christensen. 1976. Zearalenone. In: Mycotoxins in Human and Animal Health. (J.V. Rodricks, C.W. Hesseltine and M.A. Mehlman, eds.) Pathotox. Publ., Park Forest, IL, pp. 345-364
Mirocha, C.J., S.V. Pathre and T. S. Robinson. 1981. Comparative metabolism of zearalenone and transmission into bovine milk. Food Cosmet. Toxicol. 19:25.
Moy, G.G. 1998. Roles of national governments and international agencies in the risk analysis of mycotoxins. In: Mycotoxins in Agriculture and Food Safety. (K. K. Sinha and D. Bhatnagar, eds). Markel Dekker, Inc., New York, pp. 483-496.
Munkvold, G.P., R.L. Hellmich, and L.G. Rice. 1999. Comparison of fumonisin concentrations in kernels of transgenic maize Bt hybrids and nontransgenic hybrids. Plant Dis 83:130–138.
Murphy, P.A., L.G. Rice and P.F. Ross. 1993. Fumonisin Bl, B2 and B3 content of Iowa, Wisconsin and Illinois corn and corn screenings. Agric. Food Chem. 41:263-266.
Nelson, P.E., A.E. Desjardins and R.D. Plattner. 1993. Fumonisins, mycotoxins produced by Fusarium species: Biology, chemistry and significance. Ann. Rev. Phytopathol. 31:233-249.
Nibbelink, S.K. 1986. Aflatoxicosis in food animals: A clinical review. Iowa State Univ. Vet. 48:28-31.
Nip, W.K. and F.S. Chu. 1979. Fate of ochratoxin A in goats. J. Environ. Sci. Health B14:319.
Niyo, K.A., J.L. Richard, Y. Niyo, and L.H. Tiffany. 1988a. Effects of T-2 mycotoxin ingestion on phagocytosis of Aspergillus fumigatus conidia by rabbit alveolar macrophages and on hematologic, serum biochemical, and pathologic changes in rabbits. Am. J. Vet. Res. 49:1766–1773.
Niyo, K.A., J.L. Richard, Y. Niyo, and L.H. Tiffany. 1988b. Pathologic, hematologic, serologic, and mycologic changes in rabbits given T-2 mycotoxin orally and exposed to aerosols of Aspergillus fumigatus conidia. Am. J. Vet. Res. 49:2151–2160.
Noller, C.H., M. Stob, and J. Tuite. 1979. Effects of feeding Gibberella zeae-infected corn on feed intake, bodyweight gain and milk production of dairy cows. J. Dairy Sci. 62:1003-1006.
NTP (National Toxicology Program).1999. Toxicology and carcinogenesis studies on fumonisin B1 in F344/N rats and B6CF1 mice (feed studies). Technical Report Series, n 496. NIH Publication No. 99-3955. U.S. Department of Health and Human Services, National Institutes of Health Research Triangle Park, NC.
Osweiler, G.D., M.E. Kehrli, J.R. Stabel, J.R. Thurston, P.F. Ross and T.M. Wilson. 1993. Effects of fumonisin-contaminated corn screenings on growth and health of feeder calves. J. Anim. Sci. 71:459-466.
Patterson, D.S.P. and P.H. Anderson. 1982. Recent aflatoxin feeding experiments in cattle. Vet. Rec. 110:60-61.
Payne, G.A. 1983. Nature of field infection by Aspergillus flavus. In: Aflatoxin and Aspergillus flavus in Corn. (U.L Diener, R.L Asquith and J.W. Dickens, eds). Southern Coop Serv. Bull. 279. Craftmaster, Opelika, AL, pp 16-19.
Petrie, L., J. Robb and A.F. Stewart. 1977. The identification of T-2 toxin and its association with a hemorrhagic syndrome in cattle. Vet. Rec. 101:326-326.
Pier, A.C. 1992. Major biological consequences of aflatoxicosis in animal production. J. Anim. Sci. 70:3964-3970.
Pier, A.C., J.L. Richard and S.J. Cysewski. 1980. The implication of mycotoxins in animal disease. J. Am. Vet. Med. Assoc. 176:719-722.
Prelusky, D.B., P.M. Scott, H.L. Trenholm and G.A. Lawrence. 1990. Minimal transmission of zearalenone to milk of dairy cows. J. Environ. Sci. Health B25:87.
Prelusky, D.B., H.L. Trenholm, G.A. Lawrence and P.M. Scott. 1984. Nontransmission of deoxynivalenol (vomitoxin) to milk following oral administration to dairy cows. J. Environ. Sci. Health. B19:593.
Prelusky, D.B., D.M. Veira, H.L. Trenholm and B.C. Foster. 1987. Metabolic fate and elimination in milk, urine and bile of deoxynivalenol following administration to lactating sheep. J. Environ. Sci. Health. B22:125.
Prelusky, D.B., H.L. Trenholm, B.A. Rotter, J.D. Miller, M.E. Savard, J.M. Yeung, and P.M. Scott. 1996. Biological fate of fumonisin B1 in food producing animals. In: Fumonisins in Food, Vol. 392 of Advances in Experimental Medicine and Biology (L.S. Jackson, J.W. DeVries and L.B. Bullerman, eds.). Plenum Press, New York, pp. 265-278.
Puntenney, S.B., Y. Wang, and N.E. Forsberg. 2003. Mycotic infections in livestock: Recent insights and studies on etiology, diagnostics and prevention of Hemorrhagic Bowel Syndrome, In: Southwest Nutrition & Management Conference, Pheonix, University of Arizona, Department of Animal Science, Tuscon, pp. 49-63.
Radostits, O.M., G.P. Searcy, and K.G. Mitchall. 1980. Moldy sweetclover poisoning in cattle. Can. Vet. J. 21:155-158.
Raisbeck, M.F., G.E. Rottinghaus and J.D. Kendall. 1991. Effects of naturally occurring mycotoxins on ruminants. In: Mycotoxins and Animal Foods. (J.E. Smith and R.S. Henderson, eds.). CRC Press, Boca Raton, FL, pp.647-677.
Rheeder, J.P., S.F.O. Marassas, P.G. Thiel, E.W. Sydenham, G.S. Spephard and D.J. VanSchalkwyk. 1992. Fusarium moniliforme and fumonisins in corn in relation to human esophageal cancer in Transkei. Phytopathologh 82:353-357.
Richard, J.L. 1991. Mycotoxins as immunomodulators in animal systems. In: Mycotoxins, Cancer, and Health. (G.A. Bray and D.H. Ryan, eds). Pennington Center Nutrition Series, Louisiana State University Press, Baton Rouge, Louisiana. pp. 197–220.
Richard, J.L., G. Meerdink, C.M. Maragos, M. Tumbleson, G. Bordson, L.G. Rice, and P.F. Ross. 1996. Absence of detectable fumonisins in the milk of cows fed Fusarium proliferatum (Matsushima) Nirenberg culture material. Mycopathologia 133:123-126.
Riley, R.T. 1998. Mechanistic interactions of mycotoxins: Theoretical considerations. In: Mycotoxins in Agriculture and Food Safety. (K.K. Sinha and D. Bhatnagar, eds.). Marcel Dekker, Inc., New York, pp. 227–253.
Riley, R.T. and W.P. Norred. 1996. Mechanisms of mycotoxicity. In: The Mycota. Vol. VI. (D.H. Howard and J.D. Miller, eds.). Springer Verlag, Berlin, Germany, pp. 194–211.
Riley, R.T., E. Wang, J.J. Schroeder, E.R. Smith, R.D. Plattner, H. Abbas, H.-S. Yoo, and A.H. Merrill, Jr. 1996. Evidence for disruption of sphingolipid metabolism as a contributing factor in the toxicity and carcinogenicity of fumonisins. Natural Toxins 4:3–15.
Robbins, J.E., J.K. Porter, and C.W. Bacon. 1986. Occurrence and clinical manifestations of ergot and fescue toxicoses. In: Diagnosis of Mycotoxicoses. (J. L. Richard and J. L. Thurston, eds.). Martinus Nijhoff Publishers, Dordrecht, The Netherlands, pp. 61–74.
Robinson, T.S., C.J. Mirocha, H.J. Kurts, J.C. Behrens, M.S. Chi, G.A. Weaver and S.D. Nystrom. 1979. Transmission of T-2 toxin into bovine and porcine milk. J. Dairy Sci. 62:637.
Roine, K., E.L. Korpinen and K. Kallela. 1971. Mycotoxicosis as a probable cause of infertility in dairy cows. Nord. Vet. Med. 23:628-633.
Russell, T.E., T.F Watson and G.F. Ryan. 1976. Field accumulation of aflatoxin in cottonseed as influenced by irrigation termination dates and pink bollworm infestation. Appl. Environ. Microbiol. 31:711-713.
Russell, L., D.F. Cox, G. Larsen, K. Bodwell, and C.E Nelson. 1991. Incidence of molds and mycotoxins in commercial animal feed mills in seven Midwestern states, 1988-89. J. Anim. Sci. 69:5-12.
Schaeffer, J.L., and P.B. Hamilton. 1991. Interactions of mycotoxins with feed ingredients. Do safe levels exist? In: Mycotoxins and Animal Food. (J. E. Smith and R. S. Henderson, eds.). CRC Press. Boca Raton, FL, pp 827-843.
Scheideler, S.E. 1993. Effects of various types of aluminosilicates and allatoxin B1 on aflatoxin toxicity, chick performance and mineral status. Poulty Sci. 72:282-288.
Schiefer, H.B. 1990. Mycotoxicosis of domestic animals and their diagnosis. Can. J. Physiol. Pharmacol. 68:987-990.
Scott, P.M. 1990. General referee reports: Mycotoxins. J. Assoc. Off. Anal. Chem. 73:98-105.
Scott, P.M., T. Delgado, D.B. Prelusky, H.L. Trenholm, J.D. Miller. 1994. Determination of fumonisin in milk. J. Environ. Sci. Health. B29:989-998.
Seglar, B. 1997. Case studies that implicate silage mycotoxins as the cause of dairy herd problems. In: Silage: Field to Feedbunk. NRAES-99, Northeast Regional Agricultural Engineering Service, Ithaca, NY, pp. 242-254.
Shadmi, A., R. Volcani and T.A. Nobel. 1974. The pathogenic effect on animals fed mouldy hay or given its etheric fraction. Zentralbl. Veterinaermed. Reihe A. 21:544-552.
Sharma, R.P. 1993. Immunotoxicity of mycotoxins. J. Dairy Sci. 76:892-897.
Shreeve, B.J., D.S.P. Patterson and B.A. Roberts. 1979. The ‘carry-over’ of aflatoxin, ochratoxin and zearalenone from naturally contaminated feed to tissues, urine and milk of dairy cows. Fd. Cosmet. Toxicol. 17:151.
Smith, J., C. Wesselink, J. Parr, J.M. Sprosen, E.A. Fowke, N.R. Towers, and D. Laboyrie. 1995. Effect of zearalenone on ewe pregnancy rates. In: Toxinology and Food Safety. Toxinology and Food Safety Research Group, Ruakura Research Centre, Hamilton, New Zealand, pp. 41-43.
Smith, J.W., C.H. Hill and P.B. Hamilton. 1971. The effect of dietary modifications on aflatoxicosis in the broiler chicken. Poultry Sci. 50:768-771.
Smith, T.K. 1980. Influence of dietary fiber, protein and zeolite on zearalenone toxicosis in rats and swine. J. Anim. Sci. 50:278-285.
Smith, T.K. 1984. Spent canola oil bleaching clays: potential for treatment of T-2 toxicosis in rats and short term inclusion in diets for immature swine. Can. J. Anim. Sci. 64:725-732.
Smith, T.K., and E.J. MacDonald. 1991. Effect of fusaric acid on brain regional neurochemistry and vomiting behavior in swine. J. Anim. Sci. 69:2044-2049.
Sprosen, J.M. And N.R. Towers. 1995. Urinary zearalenone metabolite concentrations in herds with fertility problems. In: Toxinology and Food Safety. Toxinology and Food Safety Research Group, Ruakura Research Centre, Hamilton, New Zealand, pp. 45-46.
Sreemannarayana, O., A.A. Frohlich, T.G. Vitti, R.R. Marquart and D. Abramson. 1988. Studies of the tolerance and disposition of ochratoxin A in young calves. J. Animal Sci. 66:1703-1711.
Still, P., A.W. Macklin, W.E. Ribelin, and E.B. Smalley. 1971. Relationship of ochratoxin A to foetal death in laboratory and domestic animals. Nature 234:563-564.
Still, P., R.-D. Wei, E.B. Smalley and F.M. Strong. 1972. A mycotoxin from Penicillium roqueforti isolated from toxic cattle feed. Fed. Proc. 31(Abstr.):733.
Towers, N.R., J.M. Sprosen, and W. Webber. 1995a. Zearalenone metabolites in cycling and non-cycling cows. In: Toxinology and Food Safety. Toxinology and Food Safety Research Group, Ruakura Research Centre, Hamilton, New Zealand, pp.46-47.
Towers, N.R., C. Wesselink, E.A. Fowke, and J.M. Sprosen. 1995b. Plasma vs. urinary zearalenone concentrations as indicators of zearalenone intake. In: Toxinology and Food Safety. Toxinology and Food Safety Research Group, Ruakura Research Centre, Hamilton, New Zealand. pp. 41-41.
Trail, F., N. Mahanti, M. Rarick, R. Mehigh, S.H. Liang, R. Zhou and J. Linz. 1995. Physical and transcriptional map of an aflatoxin gene cluster in Aspergillus parasiticus and functional disruption of a gene involved early in the aflatoxin pathway. Appl. Environ. Micro.61:2665-2673.
Trenholm, H.L., B.K. Thompson, K.E. Hartin, R. Greenhalgh and A.J. McAllister. 1985. Ingestion of vomitoxin (deoxynivalenol)-contaminated wheat by nonlactating dairy cows. J. Dairy Sci. 68:1000-1005.
Trenholm, H.L., D.B. Prelusky, J.C. Young and J.D. Miller. 1988. Reducing Mycotoxins in Animal Feeds. Publication 1827E, Cat. No. A63-1827/1988E, Agriculture Canada, Ottawa.
Tuite, J., G. Shaner, G. Rambo, J. Faster and R.W. Caldwell. 1974. The Gibberella ear rot epidemics of corn in Indiana in 1965 and 1972. Cereal Sci. Today. 19:238-241.
Turner, W.B. 1978. Fungal Metabolites. Academic Press, London, U.K.
Turner, W.B. and D.C. Alderidge. 1983. Fungal Metabolites II. Academic Press, London, U.K.
Van Egmond, H.P. 1989. Aflatoxin M1: occurrence, toxicity, regulation. In: Mycotoxins in Dairy Products. (H.P.Van Egmond, ed) Elsevier Sci. Pub. Co., Ltd. New York, pp 11-55.
Vesely, D., D. Vesela and D. Adamkova. 1981. Occurrence of PR-toxin-producing Penicillium requeforti in corn silage. Vet. Med. (Preha) 26:109-115.
Vough, L.R. and I. Glick. 1993. Round bale silage. In: Silage Production from Seed to Animal. NARES-67, Northeast Regional Agricultural Engineering Service, Ithaca, NY, pp. 117-123.
Wannemacher, R.W., Jr., D.L. Brunner and H.A. Neufeld. 1991. Toxicity of trichothecenes and other related mycotoxins in laboratory animals. In: Mycotoxins and Animal Foods. (J.E. Smith and R.S. Henderson, eds.), CRC Press, Inc., Boca Raton, FL, pp. 499-552.
Weaver, G.A., H.J. Kurtz, C.J. Mirocha, F.Y. Bates, J.C. Behrens, T.S. Robison and S.P. Swanson. 1980. The failure of T-2 mycotoxin to produce hemorrhaging in dairy cattle. Can. Vet. J. 21:210-213.
Weaver, G.A., H.J. Kurtz, J.C. Behrens, T.S. Robison, B.E. Seguin, F.Y. Bates and C.J. Mirocha 1986a. Effect of zearalenone on the fertility of virgin dairy heifers. Am. J. Vet. Res. 47:1395-1397.
Weaver, G.A., H.J. Kurtz, J.C. Behrens, T.S. Robison, B.E. Seguin, F.Y. Bates and C.J. Mirocha. 1986b. Effect of zearalenone on dairy cows. Am. J. Vet. Res. 47:1826-1828.
Whitlow, L.W. 1993. Mycotoxin contamination of silages: a potential cause of poor production and health in dairy herds. In: Silage Production From Seed to Animal. NRAES-67, Northeast Regional Agricultural Engineering Service, Ithaca, NY, pp. 220-231.
Whitlow, L.W., and W.M. Hagler, Jr. 1997. Effects of mycotoxins on the animal: The producer’s perspective. In: Silage: Field to Feedbunk. NRAES-99, Northeast Regional Agricultural Engineering Service, Ithaca, NY, pp. 222-232.
Whitlow, L.W., R.L. Nebel and W.M. Hagler, Jr. 1994. The association of deoxynivalenol in grain with milk production loss in dairy cows. In: Biodeterioration Research 4. (G.C. Llewellyn, W.V. Dashek and C.E. O’Rear, eds.) Plenum Press, New York, pp. 131-139.
Whitlow, L.W., W.M. Hagler, Jr., and B.A. Hopkins. 1998. Mycotoxin occurrence in farmer submitted samples of North Carolina feedstuffs: 1989-1997. J. Dairy Sci. 81(Abstr.):1189.
Whittaker, T.B., J.W. Dickens, F.G. Giesbrecht. 1991. Testing animal feedstuffs for mycotoxins: sampling, subsampling, and analysis. In: Mycotoxins and Animal Foods. (J.E. Smith and R.S. Henderson, eds.). CRC Press, Boca Raton, FL, pp. 153-164.
Wicklow, D.T. 1983. Taxonomic features and ecological significance of sclerotia. In: Aflatoxin and Aspergillus flavus in Corn. (U.L. Deiner, R.L. Asquith and J.W. Dickens, eds). Southern Coop. Serv. Bull. 279. Craftmaster, Opelika, AL, pp. 6- 12.
Wilson, T.M., P.E. Nelson, T.B. Ryan, C.D. Rouse, C.W. Pittman, T.P. Neal, M.L. Porterfield and G.K. Saunders. 1985. Linking leukoencephalomalacia to commercial horse rations. Vet. Med. 80:63-69.
Wood, G.E. 1991. Aflatoxin M1. In: Mycotoxins and Phytoalexins. (R.P. Sharma and D.K. Salunkhe, eds.). CRC Press, Inc., Boca Raton, Florida, pp. 145-164.
Wood, G.E. 1992. Mycotoxins in foods and feeds in the United States. J. Anim. Sci. 70:3941-3949.
Wood, G.E. and M.W. Trucksess. 1998. Regulatory control programs for mycotoxin-contaminated food. In: Mycotoxins in Agriculture and Food Safety. (K.K. Sinha and D. Bhatnagar, eds.) Markel Dekker, Inc., New York, pp. 459-451.
Yoshizawa, T., C.J. Mirocha, J.C. Behrens and S.P. Swanson. 1981. Metabolic fate of T-2 toxin in a lactating cow. Fd. Cosmet. Toxicol. 19:31.
Yoshizawa, T., T. Sakamoto, Y. Ayano and C.J. Mirocha. 1982. 3'-Hydroxy T-2 and 3'-Hydroxy HT-2 toxins: New metabolites of T-2 toxin, a trichothecene mycotoxin, in animals. Agric. Biol. Chem. 46:2613.
Very informative, useful reference material about mycotoxins, cattle and dairy food products.






.jpg&w=3840&q=75)