Mycotoxins and aquaculture
Mycotoxins - a rising threat to aquaculture
The primary objective in fish nutrition is to provide a nutritionally balanced mixture of ingredients to support the maintenance, growth, reproductive performance, flesh quality and health of the animals at an acceptable cost.
The diet should also have a minimal effect on water quality and culture systems. In order to achieve these goals the diet must provide all required nutrients in the correct balance and must be formulated to keep any anti-nutritional components below concentrations that would impede the performance and health of the fish (NRC, 1993). Anti-nutritional factors can be present as natural components of feed ingredients or occur as contaminants of feedstuffs, such as mycotoxins, algal toxins, products of lipid auto-oxidation, pesticides or heavy metals. The risk of each contaminant varies among feed ingredients. Changes in diet formulation to include new ingredients or higher concentrations of existing ingredients can significantly alter the risk of contamination. Risk assessment for each contaminant should be reviewed constantly and diet composition and preventive measures adapted accordingly.
Over the last 10 years plant-based ingredients have been increasingly used in fish diets. This change is the product of increased economic/market pressures on the fish meal and oil manufacturing industries and animal feed compounders, and the drive to produce lower cost, sustainable alternatives by the aquafeed manufacturing sector (Tacon, 2004). The aquaculture sector will have no choice but to further reduce its dependency on fish meal and oil in order to sustain its growth and remain competitive. Although this change can be achieved with relative ease in omnivorous/herbivorous finfish and crustacean species, it is a great challenge in carnivorous fish. In omnivorous fish such as channel catfish (lctalurus punctatus) nutritional formulation has already developed over the last decade to include little or no animal protein.
Because plant ingredients pose a high risk of mycotoxin contamination, moving to plant protein sources in the aquafeed industry demands careful risk assessment regarding mycotoxins, as well as the development of appropriate protection strategies for fish fed contaminated feeds.
Mycotoxins are naturally occurring, toxic chemical compounds produced by filamentous fungi (molds).
Molds can infect agricultural crops, particularly cereals and oilseeds, during crop growth, harvest, storage or processing. If the conditions for fungal growth and metabolism are right, mycotoxin contamination is often the result. Thus, production of toxic metabolites can occur during the growth of the crop, during post-harvest storage or during the storage of the compounded feed. The use of more plant-based ingredients in aquafeeds enhances both the risk of introducing mycotoxins into the feed at the point of feed manufacturing, and mycotoxin production during storage of compounded feed. Through contamination with fungal spores, plant ingredients can infect the final diet with spores. If temperature and moisture in the storage environment allow for fungal growth, additional mycotoxins can be produced in between manufacturing and use of the feed.
Since conditions for fungal growth vary greatly between the field and storage, different fungal populations may result, producing cocktails of mycotoxins. This possibility must be considered when conducting a risk assessment and implementing preventive measures. Although several hundred mycotoxins are known, the mycotoxins of most concern, based on their toxicity and common occurrence, are aflatoxin, ochratoxin A, the trichothecenes (DON, T-2 toxin), zearalenone, fumonisin, and moniliformin (Table 1).
Table 1. Occurrence of key mycotoxins.
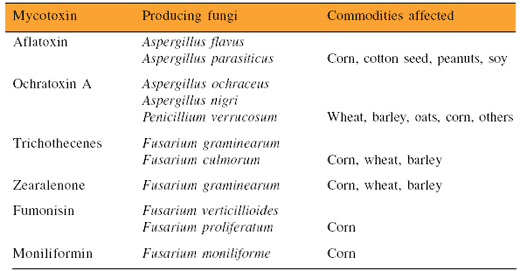
Adapted from Bhatnagar et al., 2004
Mycotoxicoses
Mycotoxins are structurally very diverse, a characteristic that leads to a wide range of symptoms in mycotoxinaffected animals. The mode of action of mycotoxins is grouped by three primary mechanisms: (1) alteration in the content, absorption and metabolism of nutrients; (2) changes in endocrine and neuroendocrine function; and, most importantly, (3) suppression of the immune system (CAST, 1989). The effects on the immune system are of particular importance as they predispose animals to infectious diseases and reduce productivity.
The fact that most of the symptoms of mycotoxicoses are rather nonspecific and can have multiple causes often makes it difficult to properly diagnose mycotoxin problems. General symptoms (reductions in performance and immune status) are seen when dealing with moderate toxin levels, while symptoms caused by higher toxin levels are more specific. Further complications in mycotoxicosis diagnoses can be caused by secondary symptoms resulting from opportunistic disease related to the suppression of the immune system after mycotoxin exposure. To effectively recognize mycotoxicosis, experience with mycotoxin-affected animals is important. This experience, combined with adequate feed and tissue analyses, provides the most accurate diagnosis of mycotoxicosis.
Aflatoxin
Aflatoxin is produced primarily by Aspergillus flavus, and is a major concern due to its carcinogenicity and ubiquity, especially in warm and humid climates. It can be produced both in the field and during storage. Due to the growth requirements of the fungi, aflatoxin poses a particular risk in warmer climates. Bautista et al. (1994) surveyed commercial shrimp feeds in the Philippines and reported aflatoxin B1 concentrations varying from 0 to 120 ppb. Surveys in Egypt showed even higher levels (>1000 ppb) for different commercial fish feeds (Abdelhamid et al., 1998).
Since aflatoxin is transferred at low rates into edible tissues, it is not only of concern for animal health, but also for the health of humans consuming food of animal origin. Therefore, in some countries the regulatory authorities have set upper limits for aflatoxin in feeds and animal products. In these markets, ingredients or diets with aflatoxins that exceed these limits must be removed and destroyed.
Aflatoxin was the first of the mycotoxins to be investigated in aquaculture. As in other animal species, aflatoxin exerts carcinogenic effects in fish. Wolf and Jackson (1963) traced hepatomes in rainbow trout exposed to increased concentrations of dietary aflatoxin B1; and exposure to low levels of aflatoxin was observed to cause hepatocellular carcinomas. Different research groups have reported that long-term exposure of <1ppb of dietary aflatoxin B1 can be sufficient to cause hepatomes (Lee et al., 1968, Sinnhuber et al., 1965).
The carcinogenic or toxic effects of aflatoxin in fish seem to be species specific. Coulombe et al. (1984) reported greater aflatoxin sensitivity in rainbow trout than in coho salmon, and noted that the ability of aflatoxin B1 to bind DNA was much greater in trout compared with salmon liver. Such differences could be linked to cytochrome P-450 metabolism of aflatoxin, which is not uniform between these species. Lower hepatotoxic effects were also reported for tilapia compared with rainbow trout (Ngethe et al., 1993).
In a 10-week feeding period, tilapia fingerlings were reported to tolerate 50 ppb aflatoxin B1 with little or no effect on performance. However, fingerlings showed a reduction in performance when feeding time was increased. A reduction in performance was observed within 10 weeks when the mycotoxin dose was raised to 100 ppb (El-Banna et al., 1992). In a second trial, with Nile tilapia, a 25-day exposure to 1880 ppb dietary aflatoxin B1 significantly reduced feed consumption and growth rate (Chavez-Sanchez et al., 1994). Normal consumption levels resumed after switching back to uncontaminated feed, although growth rate remained at a lower level, indicating long-term organ damage that affected fish were not able to overcome.
In an investigation into ‘yellow disease’ of tilapia in commercial farms in the Philippines, Cagauan et al. (2004) found that feed made with different levels of aflatoxin-contaminated corn did not significantly affect weight or weight gain of fish. However, fingerling survival was reduced by aflatoxin (P<0.001). The authors concluded that the ‘yellow disease’ was indeed the result of aflatoxin contamination of feed. Clinical signs observed in fish fed the aflatoxin-contaminated feeds were eye opacity, cataracts and blindness; skin lesions; fin and tail rot; yellowing of the body surface; abnormal swimming and reduced appetite and feeding. Histology also revealed damage or gradual deterioration of the liver.
The complexity of the task has made it impossible to determine a safe level for mycotoxins in feeds. Adverse effects depend not only on the dietary concentration, but also on the length of exposure, the fish species, the age of the fish, their nutritional status and health status.
Since aflatoxin can impair immune function (Ottinger and Kaattari, 2000), exposure increases fish susceptibility to disease. A healthy fish is less likely to succumb to secondary infections and has a greater tolerance for the toxin. Reduced immune function has been reported in Indian major carp (Labeo rohita) after exposure to aflatoxin B1 in the feed at doses as low as 1.25 mg/kg body weight (Sahoo and Mukherjee, 2001). Sahoo and Mukherjee (2002) later reported that addition of high levels of α-tocopherol in feed (1000 mg/kg) significantly improved immune response in fish exposed to aflatoxin-contaminated feed.
Aflatoxin has been shown to significantly reduce shrimp performance. Bautista et al. (1994) reported a significant reduction in performance of pre-adult shrimp (Penaeus monodon) at aflatoxin B1 concentrations of 75 ppb in a 60-day study (Figure 1). At the same level of challenge, higher susceptibility to shell diseases was also noted.
Histopathological changes in the hepatopancreas of shrimp were observed at the lowest inclusion level of 25 ppb, and became more pronounced with increasing dietary toxin concentrations. No tissue residues were detected after 60 days even at the highest inclusion level of 200 ppb, suggesting a low potential for transmission of the toxin from edible shrimp tissue to the consumer. This trial showed that typical aflatoxin concentrations found in commercial shrimp feed exceed the levels that can be tolerated without adverse effects on shrimp health and performance. A decrease in performance in response to aflatoxin B1 was reported in both Pacific white shrimp (Penaeus vannamei) and black tiger shrimp. These results differ from other measured tolerance levels of 400 to 500 ppb (Ostrowski- Meissner et al., 1995; and Boonyaratpalin et al., 2001).
In addition, Bautista and coworkers reported a decrease in diet digestibility and impaired immune function at higher aflatoxin inclusion levels.
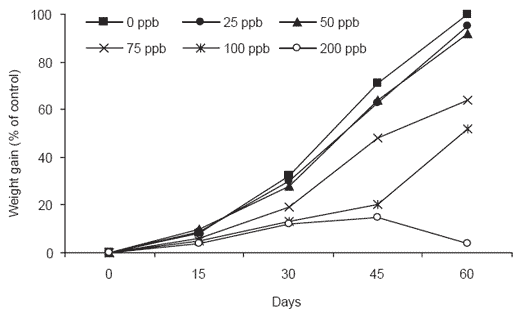
Figure 1.Percentage weight gain of shrimp fed diets contaminated with 0, 25, 50, 75, 100 and 200 ppb aflatoxin B1.
It was concluded that aflatoxin B1 concentrations commonly found in feed (Bautista et al., 1994; Abdelhamid et al., 1998), are in the range that significantly impair aquatic animal health and performance. Therefore, measures to reduce exposure must be taken when striving for maximum health, performance and economic results.
Ochratoxin A
Ochratoxin A has not been studied to the same extent as aflatoxin in aquaculture. Ochratoxin can be present in cereal grains and oilseeds, and is often formed during ingredient or diet storage. Ochratoxin A is primarily produced at higher temperatures by Penicillium verrucosum. Thus, like aflatoxin, ochratoxin is more often found in warmer climates. The key target organ of ochratoxin is the kidney, where it causes necrotic lesions in the proximal tubules.
In catfish, ochratoxin A has been shown to reduce weight gain when fed at 1000 ppb for 8 weeks (Manning et al., 2003b), although 500 ppb did not affect weight gain. Higher inclusion levels (4-8 ppm) significantly reduced feed conversion efficiency and hematocrit values. Interestingly, in contrast to observations in mammalian and avian species, the toxin did not lead to any necrotic changes in the renal tubules. Necrosis was only reported in hepato-pancreatic tissue at toxin concentrations of 1000 ppb and above.
Shalaby (2004) noted a reduction in erythrocyte count, haemoglobin concentration and haematocrit value in response to ochratoxin (at 400 and 600 μg/kg of feed) in tilapia, possibly due to destruction of mature red blood cells and inhibition of new erythrocyte production. This effect is similar to that observed in African catfish (Clarias gariepinus) exposed to ochratoxin (Mousa and Khattab, 2003). Shalaby found that inclusion of ascorbic acid at 500 mg/kg diet could offset the effects of ochratoxin. Other ochratoxin-induced effects were a significant increase in plasma glucose concentration and a dose-dependent reduction in total plasma protein, muscle and liver protein, total lipid in the plasma and a marked reduction in aminotransferase in liver and muscle.
Further research will be required to fully assess the risk ochratoxin A poses to aquaculture.
Trichothecenes and zearalenone
Trichothecenes and zearalenone are produced in temperate climates by the molds Fusarium graminearum and Fusarium culmorum. These toxins are produced in the field and enter fish diets as grain contaminants; and they continue to be produced during storage. Zearalenone has estrogen-like activity that has detrimental effects on the fertility of mammals, although it is probably of less importance in aquaculture. Arukwe et al. (1999) did report that zearalenone could affect reproductive success and the development of fish eggs, and α- zearalenone, one of its metabolites, has been shown to reduce the number and quality of sperm in carp (Sándor and Ványi, 1990).
Trichothecenes have been intensively researched, and are known to affect aquatic species. Chemically, they are structurally similar to compounds such as deoxynivalenone (DON), T2-toxin and diacetoxyscirpenol (DAS). In mammalian and avian species, trichothecenes have been shown to reduce feed intake and performance and impair immune function. In rainbow trout, Woodward et al. (1983) reported that diets containing levels of 1.0 to 12.9 ppm DON caused progressively greater reductions in 4-week live weight gain in juveniles. The depression in weight gain ranged from -12 to -92% compared with the control, and resulted from adverse effects on both feed intake and feed conversion. Complete feed refusal was observed when dietary DON concentrations reached >20 ppm.
Catfish seem more able to tolerate dietary DON (Manning, 2004). In a trial using catfish with initial body weights of 5 g no performance-reducing effect of DON at concentrations <10 ppm were observed. Only concentrations >15 ppm negatively affected performance. Such differences in species susceptibility to DON have not yet been fully investigated, and despite their tolerance to DON, catfish seem quite susceptible to T-2-toxin. Levels as low as 625 ppb have been shown to reduce catfish weight gain (Manning et al., 2003a), and higher concentrations (5000 ppb) significantly reduced feed conversion, survival rate and hematocrit concentrations. Histological inspections revealed an increased incidence of gastritis, a finding that is in agreement with intestinal lesions and gastritis reported in other species.
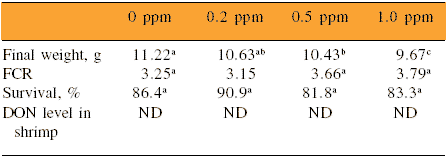
In shrimp, Trigo-Stockli et al. (2000) reported that DON concentrations as low as 0.2 ppm led to significant reductions in growth rate in the last phase of a 16-week performance trial. Overall body weight was affected at a level of 0.5 ppm (Table 2).
Toxic levels reported in both trout and shrimp seem comparable to concentrations reported in swine, where concentrations of 1 ppm or more are considered problematic, although in young piglets, lower concentrations have been shown to reduce feed intake (Spring and Strickler, 2004).
Table 2.Effect of different dietary concentrations of DON on performance of shrimp in a 16-week trial.
Fumonisin
Fumonisin is of concern to the aquaculture industry because it commonly contaminates corn and its byproducts. A survey of catfish feed ingredients in Alabama and Mississippi in the US revealed that 80% of the corn samples contained detectable levels of fumonisin. Concentrations ranged from 1.3 to 10 ppm (Lumlertdacha and Lovell, 1995). Marasas (1996) conducted a literature review of studies reporting natural occurrence of fumonisin, and noted that over 25 countries around the world had published reports detailing the natural occurrence of fumonisin in both feed and foodstuffs.
The age of animals has been reported to significantly affect the susceptibility to mycotoxicosis in different species, with young animals being more suseptible than older ones. This rule seems also to hold true for fumonisin susceptibility in catfish. Dietary fumonisin at 20 ppm has been shown to reduce growth rate in catfish with an average weight of 1.5 g (Yildirim et al., 2000). Two-year-old catfish only had reduced weight gain when exposed to 80 ppm fumonisin. Toxic effects of fumonisin have been reported in Nile tilapia at concentrations similar to catfish (Nguyen et al., 2003).
Even before performance is affected, mycotoxicosis can suppress immune function. Indeed, concentrations of 20 ppm have been shown to reduce antibody production in two-year-old catfish. These changes did not increase mortality during a challenge with Edwarsiella ictaluri, but when the catfish were exposed to 80 ppm fumonisin, resistance to the pathogenic challenge was significantly impaired (Lumlertdacha and Lovell, 1995.). Besides its effects on the immune system, fumonisin has also been shown to act as a hepatotoxic agent, and inhibits sphinganine biosynthesis (Wang et al., 1991). The sphinganine:sphingosine ratio can be used as a biomarker for fumonisin toxicoses. However, because no quick analytical test for this biomarker is available today; and it is rarely used in field situations.
Moniliformin
Moniliformin challenge trials have mainly been conducted in channel catfish. Individuals with an average initial weight of 1.5 g were fed moniliformincontaminated diets over a 10-week trial period, and results showed that they could tolerate moderate mycotoxin concentrations, but levels of 20 ppm or more significantly reduced weight gain compared with noncontaminated control diets. The diets used in this trial were semi-purified to avoid the presence of other toxins.
When feeding moniliformin in combination with fumonisin, a synergistic negative interaction between the two toxins on weight gain was observed, which is in agreement with research data from mammalian and avian species (Smith et al., 1997). Since toxins are often present as a cocktail in a single ingredient or a final diet, toxic effects due to synergistic action occur on a daily basis in the field. One has, therefore, to be careful when interpreting mycotoxin concentrations and potential risks. A concentration that does not adversely affect animal performance in a semi-purified diet can lead to problems in a natural diet in the presence of a mycotoxin cocktail.
Risk assessment and prevention strategies
Research comparing mycotoxins in different aquatic species has demonstrated that they pose a risk to fish and shrimp performance and health. The exposure to mycotoxins increases with heavier reliance on plantbased raw materials, since these ingredients pose a higher risk of mycotoxin contamination than animal-based products. With increased use of plant ingredients, mycotoxin risk assessment plans, as well as the appropriate prevention strategies, should be put in place in any aquatic production system.
Properly assessing the risk is a major challenge since it is close to impossible to define safe levels of mycotoxins. There are many factors that influence the mycotoxin concentration at which fish and shrimp will be affected in terms of health and performance. Research has shown that species differences exist in the way fish cope with mycotoxins. Both the number of known mycotoxins and the number of fish species in production have increased to large numbers today, and are poised to continue this trend over the coming years. As a consequence, it seems impossible to determine the species-specific susceptibility towards a key range of mycotoxins within a reasonable time frame.
Beside the problems related to species susceptibility, many other factors make risk assessment a great challenge. Duration of toxin exposure, age of the fish, plus their nutritional and heath status are all factors that influence how the fish or shrimp respond to a mycotoxin. Last but not least, determining the dietary mycotoxin concentrations is a great challenge by itself. Mycotoxin distribution in the feed is often uneven, so taking a representative sample is the first important step towards achieving meaningful analytical results. Analyses are limited to a number of key mycotoxins, with many being regarded as less important. Minor (or unknown) mycotoxins may not be taken into consideration.
Since mycotoxicosis risk is very difficult to judge, prevention strategies should be initiated when assessing even a low-risk situation. Prevention strategies must primarily aim at minimizing mycotoxin formation in the field and during storage. A significant reduction in mycotoxin formation can be achieved by good agronomic practices. For example, the selection of crop varieties that are more resistant to fungal foliar diseases may reduce fungal infection and thus mycotoxin formation in the standing crop. Additionally, mold spore levels have shown to be higher with no-till soil management practices and monoculture cropping systems. Proper crop rotation, including plowing up harvest residues, are two of the most effective measures to reduce mycotoxin formation in the field. As toxins are generally very stable, they can persist during storage, independent of storage conditions, and can hence reach the final feed.
During storage mold growth and mycotoxin formation can be controlled successfully by controlling moisture content of the feed. If the moisture content is below 12%, molds become metabolically inactive, and no mycotoxins are produced. The incorporation of technical mold inhibitors such as Mold-Zap (Alltech, Inc.) further enhances stability of feed and ingredients during storage. If problematic levels of mycotoxins occur despite preventative measures being taken in both field and silo, dilution or, preferably, complete removal of the contaminated ingredient is the logical solution. It must be remembered that dilution of contaminated ingredients is illegal in some markets, and it is often not practical to completely remove certain ingredients due to associated costs.
One of the most effective methods of reducing the effects of mycotoxins is the inclusion of a mycotoxinadsorption agent. This corrective action can only be taken if the mycotoxin concentrations are below legal limits (e.g., aflatoxin legal limits for raw materials in certain markets). An effective sequestering agent is one that tightly binds mycotoxins in contaminated feed without disassociating from them in the gastrointestinal tract of the animal. The toxin-sequestrant complex can then pass safely through the animal and be eliminated via the feces, minimizing animal exposure to mycotoxins.
The following guidelines should be utilized when evaluating a mycotoxin binder:
1) High level of specificity and affinity for a wide range of different mycotoxins
2) No absorption of minerals, vitamins and drugs
3) Low level of inclusion
4) Quality control (no contaminants)
5) Stability over different pH values
6) Scientifically tested in controlled in-vitro and invivo studies
One adsorbent product meeting these criteria is Mycosorb® (Alltech, Inc.), which is derived from the glucan fraction of the yeast cell wall. Yiannikouris et al. (2003) have investigated the binding of different mycotoxins by Mycosorb® in vitro, and they have developed complex models to describe the interactions between ZEA and Mycosorb®. The models give detailed information on the physical and chemical mechanisms involved in the linkage between adsorbent and toxins (detailed elsewhere in this volume).
In vitro adsorption of mycotoxins has been confirmed in vivo. For example, Pavicic and coworkers (2001) showed that Mycosorb® was able to alleviate the negative effects of DAS on weight gain in broilers (Table 3).
These effects have been confirmed in other trials with other key mycotoxins where Mycosorb® at 500 to 2000 ppm was shown to partially or completely alleviate the negative effects of the toxins on animal metabolism and performance (Raju and Devegowda, 2000; Swamy and Dewegowda, 1998; Swamy et al., 2002a,b; Raymond et al., 2003).
Table 3.Effect of a trichothecenes (DAS) and Mycosorb® on weight gain in broilers grown to six weeks of age.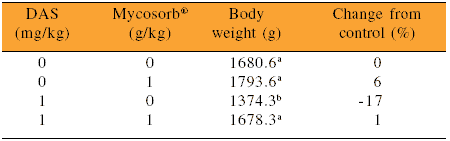
Pavicic et al., 2001
Conclusions
Plant ingredients pose a high risk for mycotoxin contamination. Since the aquafeed industry is moving towards using more plant ingredients, both risk assessment of mycotoxins as well as the development of appropriate protection strategies will become an integral part of aquaculture nutrition. Prevention strategies must target the production chain from cropping systems to animal feeding. Adsorbents that bind mycotoxins and decrease their bioavailability show a great deal of promise in strategies that attenuate mycotoxin-induced toxicosis. The high affinity and high adsorption capacity of yeast-derived glucomannan preparations make their use as adjuncts for controlling naturally occurring mycotoxins in feeds attractive.
References
Authors: PETER SPRING 1 and DANIEL F. FEGAN 2Abdelhamid, A.M., F.F. Khalil and M.A. Ragab. 1998. Problem of mycotoxins in fish production. Egyptian J. Nutr. Feeds 1:63–71.
Arukwe, A., T. Grotmol, T.B. Haugen, F.R. Knudsen and A. Goksøyr. 1999. A fish model for assessing the in vivo estrogenic potency of the mycotoxin zearalenone and its metabolites. Sci. Tot. Environ 236:153–161.
Bautista, M.N, C.R. Lavilla-Pitogo, P.F. Subosa and E.T. Begino. 1994. Aflatoxin B1 contamination of shrimp feeds and its effect on growth and hepatopancreas and pre-adult Penaeus monodom. J. Sci. Food Agri. 65:5–11.
Bhatnagar, D., G.A. Payne, T.E. Cleveland and J.F. Robens. 2004. Mycotoxins: Current issue in USA. In: Meeting the Mycotoxin Menace (D. Barug D. et al., eds). Wageningen Academic Publisher, Wageningen, NL.
Boonyaratpalin, M, K. Supamattaya, V. Verankunpiriya and D. Suprasert. 2001. Effect of aflatoxin B1 on growth and performance, blood components, immune function and histopathological changes in black tiger shrimp. Aqua. Res. 32:388-98.
Cagauan, A.G., R.H. Tayaban, J. R. Somga and R. M. Bartolome. 2004. Effect of aflatoxin-contaminated feeds in Nile tilapia (Oreochromis niloticus L.). In: Proceedings of the 6th International Symposium on Tilapia in Aquaculture (R.B. Remedios, G.C. Mair and K. Fitzsimmons, eds). Pages 172–178.
CAST. 1989. Mycotoxins: Economic and Health Risks. Council for Agriculture Science and Technology Task Force Report 116. Ames, IA.
Chavez-Sanchez, Ma.C., C.A.M. Palacios and I.O. Moreno. 1994. Pathological effects of feeding young Oreochromis niloticus diets supplemented with different levels of aflatoxin B1. Aquaculture 127:49– 60.
Coulombe, R.A., Jr., G.S. Bailey and J.E. Nixon. 1984. Comparative activation of aflatoxin B1 to mutagens by isolated hepatocytes from rainbow trout (Salmo gairdneri) and coho salmon (Onchorynchus kisutch). Carcinogenesis 5:29–33.
El-Banna, R., H.M. Teleb, M.M. Hadi and F.M Fakhry. 1992. Performance and tissue residue of tilapia fed dietary aflatoxin. Vet. Med. J. Giza 40:17–23.
Lee, D.J., J.H. Wales, J.L. Ayres and R.O. Sinnhuber. 1968. Synergism between cyclopropenoid fatty acids and chemical carcinogens in rainbow trout (Salmo gairdneri). Cancer Res. 28:2312–2318.
Lumlertdacha, S. and R.T. Lovell. 1995. Fumonisincontaminated dietary corn reduced survival and antibody production by channel catfish challenged with Edwardsiella ictaluri. J. Aquatic Anim. Health 7:1–8.
Lumlertdacha, S., R.T Lovell, R.A. Shelby, S.D. Lenz and B.W. Kemppainen. 1995. Growth, hematology and histopathology of channel catfish, Ictalurus punctatus, fed toxins from Fusarium moniliforme. Aquaculture 130:201–218.
Manning, B. 2004. Mycotoxin problems in aquaculture. Information presented all Alltech’s 2nd Aquaculture Workshop. Dunboyne, Ireland. November 29.
Manning, B.B., M.H. Li, E.H. Robinson, P.S. Gaunt, A.L. Camus and G.E. Rottinghaus. 2003a. Response of channel catfish Ictalurus punctatus to diets containing T-2 toxin. J. Aquatic Anim. Health 15:230- 239.
Manning, B.B., R.M. Ulloa, M.H. Li, E.H. Robinson and G.E. Rottinghaus. 2003b. Ochratoxin A fed to channel catfish causes reduced growth and lesions of hepatopancreatic tissue. Aquaculture 219:739–750.
Marasas, W.F. 1996. Fumonisins: history, worldwide occurrence and impact. Adv. Exp. Med. Biol. 392:1- 17.
Moussa, M.A. and Y.A. Khattab. 2003. The counteracting effect of vitamin C (L-ascorbic acid) on the physiological perturbations induced by ochratoxin intoxication in the African catfish (Clarias gariepinus). J. Egypt. Acad. Environ. Develop. (DEnvironmental Studies) 4(1):117–128.
Ngethe, S., T.E. Horsberg, E. Mitema and K. Ingebrigtsen. 1993. Species differences in hepatic concentrations of orally administered 3H-AFB-1 between rainbow trout and tilapia. Aquaculture 114:355–358.
Nguyen, A.T., B.B. Manning, R.T. Lovell and G.E. Rottinghaus. 2003. Responses of Nile tilapia (Oreochromis niloticus) fed diets containing different concentrations of moniliformin or fumonisin B1. Aquaculture 217:515–528.
NRC. 1993. Nutrient requirements of fish. National Academy Press. Washington DC. Ostrowski-Meissner, H.T., B.R. LeaMaster, E.O. Duerr and W.A. Walsh. 1995. Sensitivity of the pacific white shrimp, Penaeus vannamei, to aflatoxin B1. Aquaculture 131:155–164.
Ottinger, C.A. and S.L. Kaattari. 2000. Long-term dysfunction in rainbow trout exposed as embryos to alfatoxin B sub(1). Fish and Shellfish Immunol. 10:101–106.
Pavicic, P., P. Spring, N. Fuchs and A. Nemanic. 2001. Efficacy of esterified glucomannan to reduce the toxicity of diacetoxyscriprenol (DAS) in broiler chickens. 13th European Symposium on Poultry Nutrition, Blankenberge, Belgium. September 30 – October 4.
Raju, M.V.L.N. and G. Devegowda. 2000. Influence of esterified glucomannan on performance and organ morphology, serum biochemistry and hematology in broilers exposed to individual and combined mycotoxicosis (aflatoxin, ochratoxin and T-2 toxin). Br. Poult. Sci. 41:640–650.
Raymond, S.L., T.K. Smith and H.V.L.N. Swamy. 2003. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on feed intake, serum chemistry and hematology of horses and the efficacy of a polymeric glucomannan mycotoxin adsorbent. J. Anim. Sci. 81:2123–2130.
Sahoo, P.K. and S.C. Mukherjee. 2001. Immunosuppressive effects of aflatoxin B1 in Indian major carp (Labeo rohita). Comp. Immunol. Microbiol. Infect. Dis. 24(3):143–9.
Sahoo, P.K. and S.C. Mukherjee. 2002. Influence of high dietary α-tocopherol intakes on specific immune response, nonspecific resistance factors and disease resistance of healthy and aflatoxin B1-induced immunocompromised Indian major carp, Labeo rohita (Hamilton). Aqua. Nutr. 8(3):159–168.
Sandor, G. and A. Vanyi. 1990. Mycotoxin research in the Hungarian Central Veterinary Institute. Acta Vet. Hung. 38(1–2):61–68.
Shalaby, A.M.E. 2004. The opposing effect of ascorbic acid (vitamin C) on ochratoxin toxicity in Nile tilapia (Oreochromis niloticus). In: Proceedings of the 6th International Symposium on Tilapia in Aquaculture (R.B. Remedios, G.C. Mair and K. Fitzsimmons, eds). pages 209–221.
Sinnhuber, R.O., J.H. Wales, R.H. Engebrecht, D.L. Amend, W.D. Kray, J.L. Ayres and W.E. Ashton. 1965. Aflatoxins in cottonseed meal and hepatoma in rainbow trout. FASEB 24:627.
Smith, T.K., E.G. McMillan and J.B. Castillo. 1997. Effect of feeding blends of Fusarium mycotoxincontaminated grains containing deoxynivalenol and fusaric acid on growth and feed consumption of immature swine. J. Anim. Sci. 75(8):2184–2191.
Spring P. and B. Strickler. 2004. Effect of bedding with mycotoxin contaminated straw and low levels of dietary mycotoxin on piglet performance. 26th Mycotoxin Workshop. May 17-19. ISSN 1611-4159:S 35.
Swamy, H.V.L.N. and G. Devegowda. 1998. Ability of Mycosorb® in counteracting aflatoxicosis in commercial broilers. Indian J. Poult. Sci. 33:273– 278.
Swamy, H.V.L.N., T.K. Smith, E.J. MacDonald, H.J. Boermans and E.J. Squires. 2002a. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on swine performance, brain regional neurochemistry and serum chemistry and the efficacy of a polymeric glucomannan mycotoxin adsorbent. J. Anim. Sci. 80:3257–3267.
Swamy, H.V.L.N., T.K. Smith, P.F. Cotter, H.J. Boermans and A.E. Sefton. 2002b. Effects of feeding blends of grains naturally contaminated with Fusarium mycotoxins on production and metabolism in broilers. Poult. Sci. 81:966–975.
Tacon, A.G.J. 2004. Fish meal and fish oil use in aquaculture: global overview and prospects for substitution. In: Nutritional Biotechnology in the Feed and Food Industries, Proceedings of Alltech’s 20th Annual Symposium (T.P. Lyons and K.A. Jacques, eds). Nottingham University Press, Nottingham, UK, pp. 433–448.
Trigo-Stockli, D.M., L.O. Obaldo, W.G. Dominy and K.C. Behnke. 2000. Utilisation of DON-contaminated hard red winter wheat for shrimp feeds. J. World Aqua. Soc. 31:247–254.
Wang, E., W.P. Norred, C.W. Bacon, R.T. Riley and A.H. Merrill. 1991. Inhibition of sphingolipid biosynthesis by fumonisins. J. Biol. Chem. 266:1486- 1490.
Wolf, H. and E.W. Jackson. 1963. Hepatomas in rainbow trout: Descriptive and experimental epidemiology. Science 142:676–678.
Woodward, B., L.G. Young and A.K. Lun. 1983. Vomitoxin in diets for rainbow trout (Salmo gairdneri). Aquaculture 35:93–10.
Yiannikouris, A., L. Poughon, X. Cameleyre, C. Dussap, J. François, G. Bertin and J.P. Jouany. 2003. A novel technique to evaluate interactions between Saccharomyces cerevisiae cell wall and mycotoxins: application to zearalenone. Biotech. Lttr. 25:783–789.
Yildirim, M., B. Manning, R.T. Lovell, J.M. Grizzle and G.E. Rottinghaus. 2000. Toxicity of moniliformin and fumonisin B1 fed singly and in combination in diets for channel catfish. J. World Aqua. Soc. 31:599– 608.
1 Swiss College of Agriculture, Zollikofen, Switzerland
2 Alltech Inc., Bangkok, Thailand
Actually here we cant see the discussion about the pathogenesis of mycotoxin. also didnt discuss about the relation between mycotoxin and different types of aquaculture.






.jpg&w=3840&q=75)