Lack of Dose- and Time-Dependent Effects of Aflatoxin B1 on Gene Expression and Enzymes Associated with Lipid Peroxidation and the Glutathione Redox System in Chicken
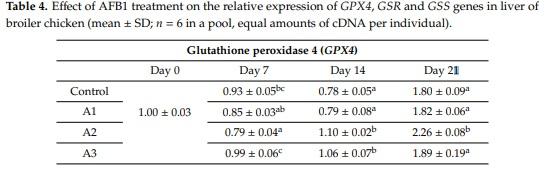
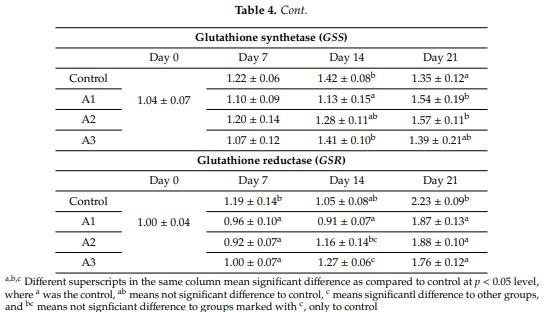
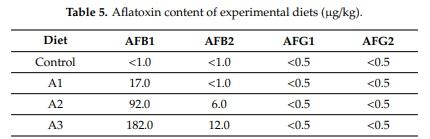
Abstract: Effects of aflatoxin B1 (AFB1) on lipid peroxidation and glutathione system were investigated in chicken liver. In a three-week feeding trial, different doses (<1.0 µg/kg (control diet), 17.0 µg (diet A1), 92.0 µg (diet A2), and 182.0 µg (diet A3) AFB1 kg/feed) were used. Markers of lipid peroxidation, conjugated dienes and trienes showed higher values in A3, while amounts of thiobarbituric acid reactive substances were increased in the A1 group at day 21. Glutathione content was lower at day 14 in Group A2. Glutathione peroxidase 4 activity was increased at days 7 and 21 in the A3 group but reduced in the A2 and A3 groups at day 14. The GPX4 gene was downregulated at day 7 in the A2 group, but overregulated at days 14 and 21, and at day 14 in the A3 group. GSS was downregulated at day 14 in the A1 group but overregulated at day 21 in A1 and A2 groups. GSR was downregulated at days 7 and 21 in all treatment groups, but on day 14, induction was observed in the A3 group. The results indicated that AFB1 did not induce dose- or time-dependent effects on the glutathione redox system and its encoding genes at the dose range used, which means that oxidative stress is not the primary effect of AFB1 toxicity.
Keywords: aflatoxin B1; glutathione redox system; broiler chicken; lipid peroxidation; gene expression.
Key Contribution: The results of the present study indicated that AFB1 at the dose range of 17 to 182 µg/kg feed did not induce lipid peroxidation and dose- or time-dependent effects on the glutathione system and its encoding genes in a three-week long trial in chicken liver. It means that oxidative stress is not the primary effect of AFB1 toxicity
1. Yunus, A.W.; Razzazi-Fazeli, E.; Bohm, J. Aflatoxin B1 in affecting broiler’s performance, immunity and
gastrointestinal tract: A review of History and Contemporary Issues. Toxins 2011, 3, 566–590. [CrossRef]
2. Dobolyi, Cs.; Seb?ok, F.; Varga, J.; Kocsubé, S.; Szigeti, G.; Baranyi, N.; Szécsi, Á.; Tóth, B.; Varga, B.; Kriszt, B.;
et al. Occurrence of aflatoxin producing Aspergillus flavus isolates in maize kernel in Hungary. Acta. Aliment.
2013, 42, 451–459. [CrossRef]
3. Battilani, P.; Toscano, P.; Fels-Klerx, H.J.; Moretti, A.; Leggieri, M.C.; Brera, C.; Rortais, A.; Goumperis, T.;
Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016,
6, 24328. [CrossRef]
4. Fountain, J.C.; Bajaj, P.; Nayak, S.N.; Yang, L.; Pandey, M.K.; Kumar, V.; Jayale, A.S.; Chitikineni, A.; Lee, R.D.;
Kemerait, R.C.; et al. Responses of Aspergillus flavus to oxidative stress are related to fungal development
regulator, antioxidant enzyme, and secondary metabolite biosynthetic gene expression. Front. Microbiol.
2016, 7, 2048. [CrossRef]
5. Ledoux, D.R.; Rottinghaus, G.E.; Bermudez, A.J.; Alonso–Debolt, M. Efficacy of hydrated sodium calcium
aluminosilicate to ameliorate the toxic effects of aflatoxin in broiler chicks. Poult. Sci. 1999, 78, 204–210.
[CrossRef] [PubMed]
6. Liu, Y.; Wang, W. Aflatoxin B1 impairs mitochondrial functions, activates ROS generation, induces apoptosis
and involves Nrf2 signal pathway in primary broiler hepatocytes. Anim. Sci. J. 2016, 87, 1490–1500.
[CrossRef] [PubMed]
7. Yarru, L.P.; Settivari, R.S.; Antoniou, E.; Ledoux, D.R.; Rottinghaus, G. Toxicological and gene expression
analysis of the impact of aflatoxin B1 on hepatic function of male broiler chicks. Poult. Sci. 2009, 88, 360–371.
[CrossRef] [PubMed]
8. Yarru, L.P.; Settivari, R.S.; Gowda, N.K.; Antoniou, E.; Ledoux, D.R.; Rottinghaus, G.E. Effects of turmeric
(Curcuma longa) on the expression of hepatic genes associated with biotransformation, antioxidant, and
immune systems in broiler chicks fed aflatoxin. Poult. Sci. 2009, 88, 2620–2627. [CrossRef] [PubMed]
9. Erdélyi, M.; Balogh, K.; Pelyhe, C.; Kövesi, B.; Nakade, M.; Zándoki, E.; Mézes, M.; Kovács, B. Changes in the
regulation and activity of glutathione redox system, and lipid peroxidation processes in short-term aflatoxin
B1 exposure in liver of laying hens. J. Anim. Physiol. Anim. Nutr. 2018, 102, 947–952. [CrossRef]
10. Salem, R.; El-Habashi, N.; Fadl, S.E.; Sakr, O.A.; Elbialy, Z.I. Effect of probiotic supplement on aflatoxicosis
and gene expression in the liver of broiler chicken. Environ. Toxicol. Pharmacol. 2018, 60, 118–127. [CrossRef]
11. Iqbal, S.Z.; Nisar, S.; Asi, M.R.; Jinap, S. Natural incidence of aflatoxins, ochratoxin A and zearalenone in
chicken meat and eggs. Food Control 2014, 43, 98–103. [CrossRef]
12. Arafa, A.S.; Bloomer, R.J.; Wilson, H.R.; Simpson, C.F.; Harms, R.H. Susceptibility of various poultry species
to dietary aflatoxin. Br. Poult. Sci. 1981, 22, 431–436. [CrossRef] [PubMed]
13. Lozano, M.C.; Diaz, G.J. Microsomal and cytosolic biotransformation of aflatoxin B1 in four poultry species.
Br. Poult. Sci. 2006, 47, 734–741. [CrossRef] [PubMed]
14. Leeson, S.; Diaz, G.J.; Summers, J.D. Poultry Metabolic Disorders and Mycotoxins; University Books: Guelph,
ON, Canada, 1995; pp. 249–280.
15. Rawal, S.; Kim, J.E.; Coulombe, R.A. Aflatoxin B1 in poultry: toxicology, metabolism and prevention. Res.
Vet. Sci. 2010, 89, 325–331. [CrossRef]
16. Bbosa, G.S.; Kity, D.; Lubega, A.; Ogwal-Okeng, J.; Anokbonggo, W.W.; Kyegomba, D.B. Review of the
biological and health effects of aflatoxins on bodyorgans and body systems. In Aflatoxins—Recent Advances
and Future Prospects; Razzaghi-Abyaneh, M., Ed.; InTech: Rijeka, Croatia, 2013; Volume 12, pp. 239–265.
[CrossRef]
17. Guengerich, F.P.; Jonhson, W.W.; Shimada, T.; Ueng, Y.F.; Yamazaki, H.; Langouet, S. Activation and
detoxication of aflatoxin B1. Mutat. Res. 1998, 402, 121–128. [CrossRef]
18. Diaz, G.J.; Murcia, H.W. Biotransformation of aflatoxin B1 and its relationship with the differential toxicological
response to aflatoxin in commercial poultry species. In Aflatoxins—Biochemistry and Molecular Biology;
Guevara-Gonzalez, R.D., Ed.; InTech: Rijeka, Croatia, 2011; Volume 1, pp. 3–20. [CrossRef]
19. IARC. Available online: http://monographs.iarc.fr/ENG/Classification/index.php (accessed on 16 September
2019).
20. Mary, V.S.; Theumer, M.G.; Arias, S.L.; Rubinstein, H.R. Reactive oxygen species sources and biomolecular
oxidative damage induced by aflatoxin B1 and fumonisin B1 in rat spleen mononuclear cells. Toxicology 2012,
302, 299–307. [CrossRef]
21. Wang, W.J.; Xu, Z.L.; Yu, C.; Xu, X.H. Effects of aflatoxin B1 on mitochondrial respiration, ROS generation
and apoptosis in broiler cardiomyocytes. Anim. Sci. J. 2017, 1561–1567. [CrossRef]
22. Abdel-Wahhab, M.A.; Abdel-Galil, M.M.; El-Lithey, M. Melatonin counteracts oxidative stress in rats fed an
ochratoxin A contaminated diet. J. Pineal. Res. 2005, 38, 130–135. [CrossRef]
23. Shi, D.Y.; Liao, S.Q.; Guo, S.N.; Li, H.; Yang, M.M.; Tang, Z.X. Protective effects of selenium on aflatoxin
B1-induced mitochondrial permeability transition, DNA damage, and histological alterations in duckling
liver. Biol. Trace Elem. Res. 2015, 163, 162–168. [CrossRef]
24. Ma, Q.; Li, Y.; Fan, Y.; Zhao, L.; Wei, H.; Ji, C.; Zhang, J. Molecular mechanisms of lipoic acid protection
against aflatoxin B1
-induced liver oxidative damage and inflammatory responses in broilers. Toxins 2015, 7,
5435–5447. [CrossRef]
25. Maurya, B.K.; Trigun, S.K. Fisetin modulates antioxidant enzymes and inflammatory factors to inhibit
aflatoxin B1 induced hepatocellular carcinoma in rats. Oxid. Med. Cell. Longev. 2016, 2016, 9. [CrossRef]
[PubMed]
26. Pál, L.; Dublecz, K.; Weber, M.; Balogh, K.; Erdélyi, M.; Szigeti, G.; Mézes, M. Effect of combined treatment
with aflatoxin B1 and T-2 toxin and metabolites on some production traits and lipid peroxide status parameters
of broiled chickens. Acta. Vet. Hung. 2009, 57, 75–84. [CrossRef] [PubMed]
27. Shen, H.M.; Shi, C.Y.; Shen, Y.; Ong, C.N. Detection of elevated reactive oxygen species level in cultured rat
hepatocytes treated with aflatoxin B1. Free Radic. Biol. Med. 1996, 21, 139–146. [CrossRef]
28. Wójtowicz-Chomicz, K.; Stadnik, A.; Kowal, M.; Sztanke, K.; Sztanke, M.; Borzecki, A. Disturbances of
anti-oxidative balance in rats caused by aflatoxin B1. Bull. Vet. Inst. Pulawy 2011, 55, 145–148.
29. Gloire, G.; Legrand-Poels, S.; Piette, J. NF-kappa B activation by reactive oxygen species: Fifteen years later.
Biochem. Pharmacol. 2006, 72, 1493–1505. [CrossRef] [PubMed]
30. Suzuki, M.; Otsuki, A.; Lukwete, N.K.; Yamamoto, M. Overview of redox regulation by Keap1–Nrf2 system
in toxicology and cancer. Curr. Opin. Toxicol. 2016, 1, 29–36. [CrossRef]
31. Jobbagy, S.; Vitturi, D.A.; Salvatore, S.R.; Turell, L.; Pires, M.F.; Kansanen, E.; Batthyany, C.; Lancaster, J.R.;
Freeman, B.A.; Schopfer, F.J. Electrophiles modulate glutathione reductase activity via alkylation and
upregulation of glutathione biosynthesis. Redox. Biol. 2018, 21, 101050. [CrossRef]
32. Shelly, C.; Lu, M.D. Glutathione synthesis. Biochim. Biophys. Acta. 2013, 1830, 3143–3153. [CrossRef]
33. Imlay, J.A. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 2008, 77,
755–776. [CrossRef]
34. Biomin World Mycotoxin Survey. Annual Report No. 15. Available online: https:
//www.biomin.net/en/articles/biomin-world-mycotoxin-survey-report-2018/?utmsource=AAF&utm_
medium=Advertorial&utm_campaign=MTXSurvey (accessed on 24 October 2019).
35. Diaz, G.J.; Calabrese, E.; Blain, R. Aflatoxicosis in chickens (Gallus gallus): An example of hormesis? Poult.
Sci. 2008, 87, 727–732. [CrossRef]
36. Patterson, D.S.P. Aflatoxin and related compounds: Introduction. In Mycotoxic Fungi, Mycotoxins,
Mycotoxicoses, An Encyclopaedic Handbook, 1st ed.; Wyllie, T.D., Morehouse, L.G., Eds.; Marcel Dekker:
New York, NY, USA, 1977; Volume 1, pp. 131–135.
37. Verma, J.; Johri, T.S.; Swain, B.K.; Ameena, S. Effect of graded levels of aflatoxin, ochratoxin and their
combinations on the performance and immune response of broilers. Br. Poult. Sci. 2004, 45, 512–518.
[CrossRef] [PubMed]
38. Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482,
419–425. [CrossRef] [PubMed]
39. Yang, J.; Bai, F.; Zhang, K.; Bai, S.; Peng, X.; Ding, X.; Li, Y.; Zhang, J.; Zhao, L. Effects of feeding corn naturally
contaminated with aflatoxin B1 and B2 on hepatic functions of broilers. Poult. Sci. 2012, 91, 2792–2801.
[CrossRef] [PubMed]
40. Ali Rajput, S.; Sun, L.; Zhang, N.; Mohamed Khalil, M.; Gao, X.; Ling, Z.; Zhu, L.; Khan, F.A.; Zhang, J.; Qi, D.
Ameliorative effects of grape seed proanthocyanidin extract on growth performance, immune function,
antioxidant capacity, biochemical constituents, liver histopathology and aflatoxin residues in broilers exposed
to aflatoxin B1
. Toxins 2017, 9, 371. [CrossRef] [PubMed]
41. Khanian, M.; Karimi-Torshizi, M.A.; Allameh, A. Alleviation of aflatoxin-related oxidative damage to liver
and improvement of growth performance in broiler chickens consumed Lactobacillus plantarum 299v for
entire growth period. Toxicon 2019, 158, 57–62. [CrossRef] [PubMed]
42. Surai, P.F.; Dvorska, J.E. Effects of mycotoxins on antioxidant status and immunity. In The Mycotoxin Blue
Book; Diaz, D.E., Ed.; Nottingham University Press: Nottingham, UK, 2005; pp. 93–137.
43. Valdivia, A.G.; Martinez, A.; Damian, F.J.; Quezada, T.; Ortiz, R.; Martinez, C.; Llamas, J.; Rodriguez, M.L.;
Yamamoto, L.; Jaramillo, F.; et al. Efficacy of N-acetylcysteine to reduce the effects of aflatoxin B1 intoxication
in broiler chickens. Poult. Sci. 2001, 80, 727–734. [CrossRef]
44. Muhammad, I.; Wang, H.; Sun, X.; Wang, X.; Han, M.; Lu, Z.; Cheng, P.; Hussain, M.A.; Zhang, X. Dual role
of dietary curcumin through attenuating AFB1-induced oxidative stress and liver injury via modulating
liver phase-I and phase-II enzymes involved in AFB1 bioactivation and detoxification. Front. Pharmacol.
2018, 9, 554. [CrossRef]
45. Gowda, N.K.S.; Ledoux, D.R.; Rottinghaus, G.E.; Bermudez, A.J.; Chen, Y.C. Efficacy of turmeric, containing
a known level of curcumin, and a hydrated sodium calcium aluminosilicate to ameliorate the adverse effects
of aflatoxin in broiler chicks. Poult. Sci. 2008, 87, 1125–1130. [CrossRef]
46. Huang, J.Q.; Li, D.L.; Zhao, H.; Sun, L.H.; Xia, X.J.; Wang, K.N.; Luo, X.; Lei, X.G. The selenium deficiency
disease exudative diathesis in chicks is associated with down-regulation of seven common selenoprotein
genes in liver and muscle. J. Nutr. 2011, 141, 1605–1610. [CrossRef]
47. Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: molecular pathways and physiological
roles. Physiol. Rev. 2014, 94, 739–777. [CrossRef]
48. Hungarian Feed Code. Nutrient Requirements of Farm Animals; OMMI: Budapest, Hungary, 2004; Vol. II/II,
pp. 258–263. (In Hungarian)
49. Khayoon, W.S.; Saad, B.; Yan, C.B.; Hashim, N.H.; Ali, A.S.M.; Salleh, M.I.; Salleh, B. Determination of
aflatoxins in animal feeds by HPLC with multifunctional column clean-up. Food Chem. 2010, 118, 882–886.
[CrossRef]
50. Association of the Official Analytical Chemists (AOAC). Official Methods of Analysis 28054 B, 14th ed.; AOAC:
Arlington, VA, USA, 1984; Vol. I, pp. 1013–1015.
51. Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid,
sensitive and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food and
feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [CrossRef]
52. Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione
disulphide levels using enzymatic recycling method. Nat. Protoc. 2007, 1, 3159–3165. [CrossRef] [PubMed]
53. Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium deficient rat liver. Biochem. Biophys.
Res. Commun. 1976, 71, 952–956. [CrossRef]
54. Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent.
J. Biol. Chem. 1951, 193, 265–275.