First Report of the Production of Mycotoxins and Other Secondary Metabolites by Macrophomina phaseolina (Tassi) Goid. Isolates from Soybeans (Glycine max L.) Symptomatic with Charcoal Rot Disease
1 Department of Biochemistry, Molecular Biology, Entomology, and Plant Pathology, Mississippi State University, Mississippi State, MS 39762, USA; vhk4@msstate.edu (V.H.K.); MariaT@pss.msstate.edu (M.T.-P.) 2 Biological Control of Pests Research Unit, US Department of Agriculture, Agricultural Research Service, Stoneville, MS 38776, USA 3 Institute of Bioanalytics and Agro-Metabolomics, Department of Agrobiotechnology, IFA-Tulln, University of Natural Resources and Life Sciences, Vienna (BOKU), Konrad-Lorenz-Str. 20, Tulln 3430, Austria; michael.sulyok@boku.ac.at 4 Department of Medicinal Chemistry, College of Pharmacy, University of Minnesota, Minneapolis, MN 55455, USA.
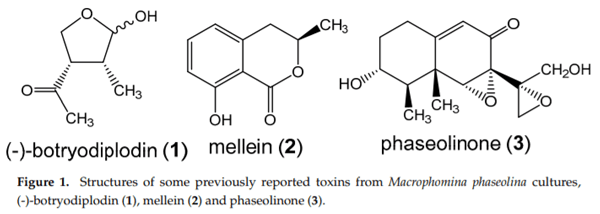
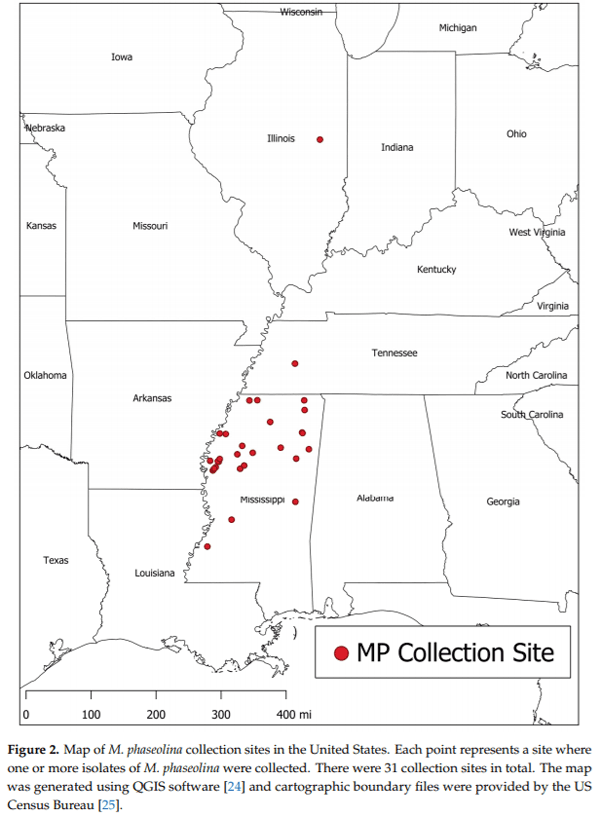
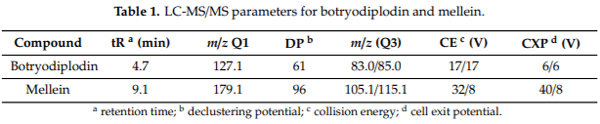
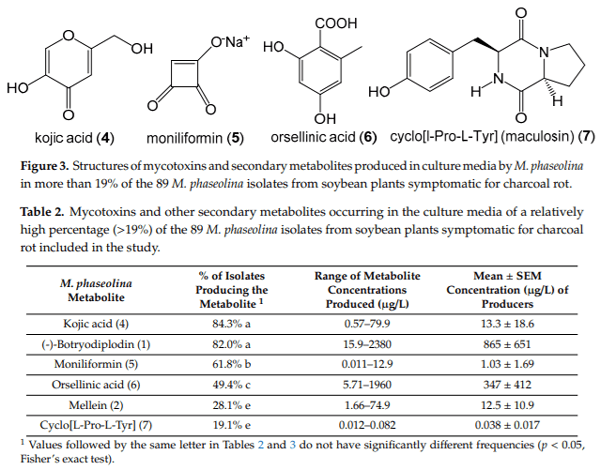
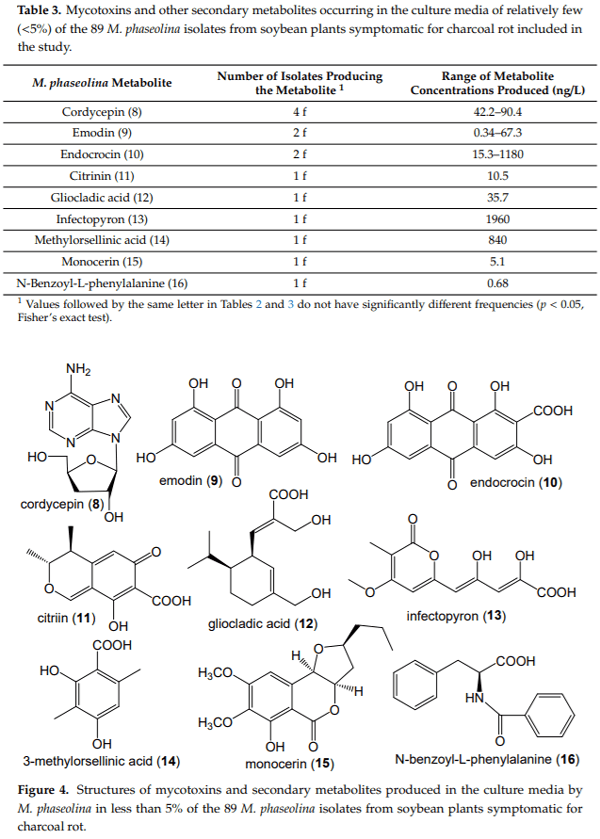
Macrophomina phaseolina (Tassi) Goid., the causal agent of charcoal rot disease of soybean, is capable of causing disease in more than 500 other commercially important plants. This fungus produces several secondary metabolites in culture, including (-)-botryodiplodin, phaseolinone and mellein. Given that independent fungal isolates may differ in mycotoxin and secondary metabolite production, we examined a collection of 89 independent M. phaseolina isolates from soybean plants with charcoal rot disease using LC-MS/MS analysis of culture filtrates. In addition to (-)-botryodiplodin and mellein, four previously unreported metabolites were observed in >19% of cultures, including kojic acid (84.3% of cultures at 0.57–79.9 µg/L), moniliformin (61.8% of cultures at 0.011–12.9 µg/L), orsellinic acid (49.4% of cultures at 5.71–1960 µg/L) and cyclo[L-proline-L-tyrosine] (19.1% of cultures at 0.012–0.082 µg/L). In addition, nine previously unreported metabolites were observed at a substantially lower frequency (<5% of cultures), including cordycepin, emodin, endocrocin, citrinin, gliocladic acid, infectopyron, methylorsellinic acid, monocerin and N-benzoyl-L-phenylalanine. Further studies are needed to investigate the possible effects of these mycotoxins and metabolites on pathogenesis by M. phaseolina and on food and feed safety, if any of them contaminate the seeds of infected soybean plants.
Keywords: moniliformin; kojic acid; mellein; orsellinic acid; cyclo[L-proline-L-tyrosine]; cordycepin; phaseolinone; botryodiplodin; natural products; phytotoxins; secondary metabolites; fungi.
- Singh, G. The Soybean: Botany, Production and Uses; Centre for Agriculture and Bioscience International (CABI): Wallingford, UK, 2010; ISBN 978-1-84593-644-0.
- United States Department of Agriculture: National Agricultural Statistics Service (USDA-NASS). Available online: https://www.nass.udsa.gov (accessed on 29 August 2020).
- Allen, T.W.; Bradley, C.A.; Sisson, A.J.; Byamukama, E.; Chilvers, M.I.; Coker, C.M.; Collins, A.A.; Damicone, J.P.; Dorrance, A.E.; Dufault, N.S.; et al. Soybean yield loss estimates due to diseases in the United States and Ontario, Canada, from 2010 to 2014. Plant Health Prog. 2017, 18, 19–27. [CrossRef]
- Hartman, G.L.; Rupe, J.C.; Sikora, E.J.; Domier, L.L.; Davis, J.A.; Ste ey, K.L. Compendium of Soybean Diseases and Pests, 5th ed.; American Phytopathological Society Press: St. Paul, MN, USA, 2015.
- Heatherly, L.G. Soybean Yield Loss to Diseases in the Midsouthern US, 2012–2019; Mississippi Soybean Promotion Board: Starkville, MS, USA, 2020.
- Ghosh, T.; Biswas, M.K.; Guin, C.; Roy, P. A review on characterization, therapeutic approaches and pathogenesis of Macrophomina phaseolina. Plant Cell Biotechnol. Mol. Biol. 2018, 19, 72–84.
- Mengistu, A.; Ray, J.D.; Smith, J.R.; Paris, R.L. Charcoal rot disease assessment of soybean genotypes using a colony-forming unit index. Crop Sci. 2007, 47, 2453–2461. [CrossRef]
- Wyllie, T.D. Macrophomina phaseolina—Charcoal rot. In World Soybean Research: Proceedings of the World Soybean Research Conference; Hill, L.D., Ed.; Interstate Printers and Publishers Inc.: Danville, IL, USA, 1976; pp. 482–484.
- Bellaloui, N.; Mengistu, A.; Paris, R.L. Soybean seed composition in cultivars di ering in resistance to charcoal rot (Macrophomina phaseolina). J. Agric. Sci. 2008, 146, 667–675. [CrossRef]
- Bellaloui, N.; Mengistu, A.; Zobiole, L.H.S.; Shier, W.T. Resistance to toxin-mediated fungal infection: Role of lignins, isoflavones, other seed phenolics, sugars, and boron in the mechanism of resistance to charcoal rot disease in soybean. Toxin Rev. 2012, 31, 16–26. [CrossRef]
- Bowen, C.R.; Schapaugh, W.T. Relationships among charcoal rot infection, yield, and stability estimates in soybean blends. Crop Sci. 1989, 29. [CrossRef]
- Gupta, G.K.; Sharma, S.K.; Ramteke, R. Biology, epidemiology and management of the pathogenic Fungus Macrophomina phaseolina (Tassi) Goid with special reference to charcoal rot of soybean (Glycine max (L.) Merrill). J. Phytopathol. 2012, 160, 167–180. [CrossRef]
- Wrather, J.A.; Koenning, S.R. Estimates of disease e ects on soybean yields in the United States 2003 to 2005. J. Nematol. 2006, 38, 173–180.
- Lodha, S.; Mawar, R. Population dynamics of Macrophomina phaseolina in relation to disease management: A review. J. Phytopathol. 2019, 168, 1–17. [CrossRef]
- Dhingra, O.D.; Sinclair, J.B. Variation among isolates of Macrophomina phaseoli (Rhizoctonia bataticola) from the same soybean plant. Phytopathology 1972, 62, S1108.
- Jana, T.K.; Singh, N.K.; Koundal, K.R.; Sharma, T.R. Genetic differentiation of charcoal rot pathogen, Macrophomina phaseolina, into specific groups using URP-PCR. Can. J. Microbiol. 2005, 51, 159–164. [CrossRef] [PubMed]
- Mayék-Pérez, N.; López-Castañeda, C.; González-Chavira, M.; Garcia-Espinosa, R.; Acosta-Gallegos, J.; de la Vega, O.M.; Simpson, J. Variability of Mexican isolates of Macrophomina phaseolina based on pathogenesis and AFLP genotype. Physiol. Mol. Plant Pathol. 2001, 59, 257–264. [CrossRef]
- Reyes-Franco, M.C.; Hernández-Delgado, S.; Beas-Fernández, R.; Medina-Fernández, M.; Simpson, J.; Mayek-Pérez, N. Pathogenic and genetic variability within Macrophomina phaseolina from Mexico and other countries. J. Phytopathol. 2006, 154, 447–453. [CrossRef]
- Ramezani, M.; Shier, W.T.; Abbas, H.K.; Tonos, J.L.; Baird, R.E.; Sciumbato, G.L. Soybean charcoal rot disease fungus Macrophomina phaseolina in Mississippi produces the phytotoxin (-)-botryodiplodin but no detectable phaseolinone. J. Nat. Prod. 2007, 70, 128–129. [CrossRef] [PubMed]
- Abbas, H.K.; Bellaloui, N.; Accinelli, C.; Smith, J.R.; Shier, W.T. Toxin production in soybean (Glycine max L.) plants with charcoal rot disease and by Macrophomina phaseolina, the fungus that causes the disease. Toxins 2019, 11, 645. [CrossRef]
- Abbas, H.K.; Bellaloui, N.; Butler, A.M.; Nelson, J.L.; Abou-Karam, M.; Shier, W.T. Phytotoxic responses of soybean (Glycine max L.) to botryodiplodin, a toxin produced by the charcoal rot disease fungus, Macrophomina phaseolina. Toxins 2020, 12, 25. [CrossRef]
- Dhar, T.K.; Siddiqui, K.A.I.; Ali, E. Structure of phaseolinone, a novel phytotoxin from Macrophomina phaseolina. Tetrahedron Lett. 1982, 23, 5459–5462. [CrossRef]
- Khambhati, V.H.; Abbas, H.K.; Shier, W.T.; Tomaso-Peterson, M.; Chen, J.; Kotowicz, J.K.; Bellaloui, N.; Mengistu, A. Identifying secondary metabolites produced by charcoal rot disease fungus, Macrophomina phaseolina, and their role in pathogenesis. J. Miss. Acad. Sci. 2020, 65, 43.
- QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available online: https://qgis.org/en/site/ (accessed on 1 November 2020).
- US Census Bureau. Cartographic Boundary Files—Shapefile. 2018. Available online: https://www.census.gov/geographies/mapping-files/time-series/geo/carto-boundary-file.html (accessed on 1 November 2020).
- Sulyok, M.; Stadler, D.; Steiner, D.; Krska, R. Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantification of > 500 mycotoxins and other secondary metabolites in food crops: Challenges and solutions. Anal. Bioanal. Chem. 2020, 412, 2607–2620. [CrossRef]
- SANTE/12089/2016. Guidance Document on Identification of Mycotoxins in Food and Feed; European Commission Directorate General for Health and Food Safety: Brussels, Belgium, 2016, Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/cs_contaminants_sampling_guid-doc-ident-mycotoxins.pdf (accessed on 28 September 2020).
- Shier, W.T.; Abbas, H.K.; Baird, R.E.; Ramezani, M.; Sciumbato, G.L. (-)-Botryodiplodin, a unique ribose-analog toxin. Toxin Rev. 2007, 26, 343–386. [CrossRef]
- Beélik, A. Kojic acid. In Advances in Carbohydrate Chemistry; Wolfrom, M.L., Tipson, R.S., Eds.; Academic Press: Cambridge, MA, USA, 1956; Volume 11, pp. 145–183. [CrossRef]
- Cabanes, J.; Chazarra, S.; Garcia-Carmona, F. Kojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinase. J. Pharm. Pharmacol. 1994, 46, 982–985. [CrossRef] [PubMed]
- Burdock, G.A.; Soni, M.G.; Carabin, I.G. Evaluation of health aspects of kojic acid in food. Regul. Toxicol. Pharmacol. 2001, 33, 80–101. [CrossRef] [PubMed]
- Wei, C.I.; Huang, T.S.; Fernando, S.Y.; Chung, K.T. Mutagenicity studies of kojic acid. Toxicol. Lett. 1991, 59, 213–220. [CrossRef]
- Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [CrossRef]
- Cole, R.J.; Kirksey, J.W.; Cutler, H.G.; Doubnik, B.L.; Peckham, J.C. Toxin from Fusarium moniliforme: E ects on plants and animals. Science 1973, 179, 1324–1326. [CrossRef]
- McLean, M. The phytotoxicity of Fusarium metabolites: An update since 1989. Mycopathologia 1996, 133, 163–179. [CrossRef]
- Hallas-Møller, M.; Nielsen, K.F.; Frisvad, J.C. Production of the Fusarium mycotoxin moniliformin by Penicillium melanoconidium. J. Agric. Food Chem. 2016, 64, 4505–4510. [CrossRef]
- Abbas, H.K.; Mirocha, C.J.; Vesonder, R.E.; Gunther, R. Acute toxic effects of an isolate of moniliformin-producing Fusarium oxysporum and purified moniliformin on rats. Arch. Environ. Contam. Toxicol. 1990, 19, 433–436. [CrossRef]
- Burmeister, H.R.; Ciegler, A.; Vesonder, R.F. Moniliformin, a metabolite of Fusarium moniliforme NRRL 6322: Purification and toxicity. Appl. Environ. Microbiol. 1979, 37, 11–13. [CrossRef]
- Thiel, P.G. A molecular mechanism for the toxic action of moniliformin, a mycotoxin produced by Fusarium moniliforme. Biochem. Pharmacol. 1978, 27, 483–486. [CrossRef]
- Birkinshaw, J.; Gowlland, A. Studies in the biochemistry of micro-organisms. 110. Production and biosynthesis of orsellinic acid by Penicillium madriti G. Smith. Biochem. J. 1962, 84, 342–347. [CrossRef] [PubMed]
- Van Eijk, G.W. Isolation and identification of orsellinic acid and penicillic acid produced by Penicillium fennelliae Stolk. Antonie Leeuwenhoek 1969, 35, 497–504. [CrossRef] [PubMed]
- Reveglia, P.; Masi, M.; Evidente, A. Melleins—Intriguing natural compounds. Biomolecules 2020, 10, 772. [CrossRef] [PubMed]
- Nishikawa, H. Biochemistry of filamentous fungi. II: A metabolic product of Aspergillus melleus Yukawa. Part I and Part II. J. Agric. Chem. Soc. Jpn. 1933, 9, 107–109, 148–151. [CrossRef]
- Salvatore, M.M.; Félix, C.; Lima, F.; Ferreira, V.; Naviglio, D.; Salvatore, F.; Duarte, A.S.; Alves, A.; Andolfi, A.; Esteves, A.C. Secondary metabolites produced by Macrophomina phaseolina isolated from Eucalyptus globulus. Agriculture 2020, 10, 72. [CrossRef]
- Zaher, A.M.; Moharram, A.M.; Davis, R.; Panizzi, P.; Makboul, M.A.; Calderón, A.I. Characterisation of the metabolites of an antibacterial endophyte Botryodiplodia theobromae Pat. of Dracaena draco L. by LC-MS/MS. Nat. Prod. Res. 2015, 29, 2275–2281. [CrossRef]






_1.jpg&w=3840&q=75)