Introduction
Mycotoxins are toxic substances produced by some fungi that are widely present in feedstuffs. They are responsible for considerable losses and adverse effects on animal health and production (Hauschild et al., 2007). Zearalenone (ZEA) is a non-steroidal estrogenic mycotoxin (Andretta et al., 2008; Andretta et al., 2010) produced mainly by Fusarium graminearum and Fusarium culmorum in corn (Fink-Gremmels and Malekinejad, 2007; Chatopadhyay et al., 2012; Gajecka et al., 2016). Once ingested, ZEA is absorbed in the gastrointestinal tract and biotransformed mainly in the liver into two metabolites, α- and β-zearalenol (Teixeira et al., 2011; Mostrom, 2012), which causes reproductive disorders and hyperestrogenic effects by binding to estrogenic receptors (Doll and Danicke, 2011). Hyperestrogenism is characterized by redness of the vulva, increased vulvar volume and possible rectal or vaginal prolapse due to relaxed sphincters. At the same time, it can cause metaplasia in the uterus, vagina and mammary glands (Bryden, 2012; Oliver et al., 2012)
Antimycotoxin additives (AMA) represent a potential new strategy to reduce the adverse effects of ZEA toxicity. Saccharomyces cerevisiae cell walls (YCW) can adsorb various mycotoxins from animal feeds (Huwig et al., 2001; Shetty and Jespersen, 2006; Arrieta et al., 2008). Cell walls of S. cerevisiae consist of 90% polysaccharides, of which mannanoligosaccharides (MOS) and β-glucans are the main structural polymers (Brady et al., 1994). The structural diversity of these cell walls reflect the presence of a wide variety of potential sites where molecular complexes can be formed (Zouboulis et al., 2001). The beneficial properties of YCWs are strain-dependent and cannot be extrapolated to the genus or species; therefore, the evaluation of any new strain (or derived product) is necessary.
Few studies have reported the chemical composition of the additive, which is a very important information in this study, since the effect of mycotoxin adsorption is associated with the levels of glucans and mannans in the additive. To the best of our knowledge, the present study is the first to compare alternative approaches on tackling/reducing the toxic effect of mycotoxins in swine farming using orthogonal contrasts. Orthogonal contrast is a linear combination of treatment means, and is a more flexible method as differences among specific levels or the combination of different treatment levels can be evaluated. Thus, the present study aimed to evaluate the efficacy of using a YCW-based commercial product (Safwall®) for preventing the toxic effects of ZEA.
Materials and methods
Ethical considerations
All procedures involving animals were in accordance with the ethical standards of the institution in which the study was conducted (Protocol 367/2013, July 11, 2013).
Antimycotoxin additive (AMA) Safwall®
Safwall® (Lesaffre do Brasil Produtos Alimentícios Ltda, São Paulo, Brazil) was the YCWbased commercial AMA tested. The additive is free of antibiotic residues, heavy metals, chemical products or microbial contaminants and has the following chemical composition: A maximum of 5% moisture and 28% protein; a minimum of 1% phosphorus, 23% β-glucans, 21% MOS, 95% dry matter, 20% fat and a maximum of 4% ash. Its physical composition is characterized by a golden cream color, typical yeast odor, and no visible impurities. The level of inclusion was the maximum dose recommended by the manufacturer (i.e. 2 g/Kg or 0.2%).
The ZEA is produced by controlled fermentation according to the method described by Jiménez et al. (1996) using polished white rice as a substrate. The organism (fungi) used in this study was a strain of F. graminearum UNC 3639 (obtained from Universidad Nacional de Córdoba, Argentina). It was cultured in a potato dextrose agar (PDA) nutrient agar medium in Petri dishes. The fungal spores collected from the culture were suspended in distilled water which was added to the rice. The ZEA matrix was autoclaved and dried for 30 d at 50 °C in a drying oven, after which it was grounded. The ZEA was extracted using a solution of distilled water and acetonitrile (Vetec, Duque de Caxias, RJ, Brasil), the extracted ZEA was purified using a MycoSep® 226 AflaZON+ (Romer Labs® Diagnostic GmbH, Tulln, Austria) extraction column according to the manufacturer›s protocol and quantified by high performance liquid chromatography (HPLC; Jasco Inc., Easton, MD, USA). The ZEA matrix was added to the basal diet using a Y-type mixer to attain 0.25 and 0.6 ppm ZEA in the experimental feed. The ZEA matrix was produced in a laboratory at Universidade Federal Rural do Rio de Janeiro (UFRRJ), Seropédica, Rio de Janeiro, Brasil.
Animals
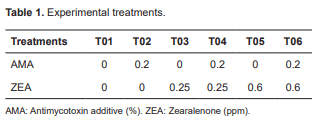
A total of 36 pre-pubertal gilts (Topig lineage) with 34 d initial age and 8 ± 0.48 Kg were used. The animals were kept in suspended stalls - 2 animals/ stall. The study was completely randomized with six treatments and 3 replicates/treatment. Each stall was considered an experimental unit. Animals were subjected to a 7-d adaptation period and a 21-d experimental period. The feed was prepared with and without AMA supplementation (0 and 0.2%) and three levels of ZEA supplementation (0, 0.25, and 0.6 ppm), as shown in Table 1.
All animals received water and feed ad libitum. The animals were observed three times daily throughout the experimental period. Ambient temperature was monitored daily. The following parameters were measured and evaluated during the experiment: Live weight (LW), weight gain (WG), feed intake (FI), and vulvar volume using a digital caliper at d 7, 14, and 21. Three animals from each treatment group were fasted for approximately 6 h at the end of the study. During this time, they were only allowed access to water; after which they were electro-stunned, and then slaughtered in accordance with humane slaughter principles. Carcasses were eviscerated and the entire reproductive tract, including the uterus-ovarian-vagina set in addition to the liver were weighed and dissected. The study model followed the recommendations of Ordinance no. 130 of May 24, 2006 by the Brazilian Ministry of Agriculture, Livestock and Food Supply (Brazil, 2006). The in vivo test was conducted at Universidade Federal Rural do Rio de Janeiro (UFRRJ), located at Seropédica/Rio de Janeiro, Brazil.
Statistical analysis
Due to the varying characteristics of the data, the standard ANOVA model was used to compare the relative weight of the organs. The following statistical model was used:
Yijk = μ+ AMAj + ZEAk + AMA * ZEAjk + eijk
Where:
Yijk: Is the value of the response to the observation of the i-th repetition of the j-th level of the factor AMA, k-th level of the factor ZEA.
µ: Is the overall mean
AMA: Is the fixed effect of the use of AMA (0 or 0.2%).
ZEA: Is the fixed effect of the use of ZEA (0, 0.25, or 0.6 ppm).
AMA * ZEA: Is the effect of AMA and ZEA interaction.
eijk: Is the random error.
The repeated measures of the ANOVA model was used for the data collected over time. The model considers the effect of time, which if not properly controlled, could mask the effects of the experimental factors (ZEA and AMA) and ultimately affect the results.
The following statistical model was used:
Yijkl = μ+ AMAj + ZEAk +Tl + AMA * ZEAjk + AMA * Tjl + ZEA * Tkl + AMA * ZEA * Tjkl + eijkl
Where:
Yijk: Is the value of the response to the observation of the i-th repetition of the j-th level of the factor AMA, k-th level of the factor ZEA and i-th moment in time.
µ: Is the overall mean.
AMA: Is the fixed effect of the use of AMA (0 or 0.2%).
ZEA: Is the fixed effect of the use of ZEA (0, 0.25, or 0.6 ppm).
T: Is the fixed effect of time (1, 7, 14, or 21 d).
AMA * ZEA: Is the effect of AMA and ZEA interaction.
AMA * T: Is the effect of AMA and TIME interaction.
ZEA * T: Is the effect of ZEA and TIME interaction.
AMA * ZEA * T: Is the effect of AMA, ZEA, and TIME interaction.
eijk: Is the random error.
In both cases, the complete model was tested, which implies that all the variables and all possible interactions were tested in the experiment. To determine the levels of statistical significance of various interactions with the factor of interest (AMA), the Student t test was applied using orthogonal contrasts to determine which cases of ZEA intoxication and/or specific treatment duration were statistically significant in relation to the use of AMA as a prophylactic for mycotoxicosis.
For non-parametric data (vulvar volume), it was necessary to transform the variable using a logarithmic function to ensure that the resulting data would conform to normality. All statistical tests considered a 5% level of significance and the analyses were conducted using the PROC MIXED program of SAS®, version 9.1 (2003, SAS Institute Inc., Cary, NC, USA).
Results
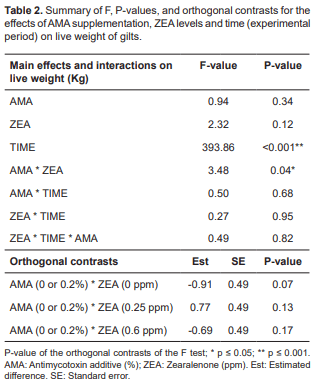
Regarding live weight (Table 2), there was only a significant effect of time, increasing live weight as weeks passed (1, 7, 14, and 21 d; p<0.001).
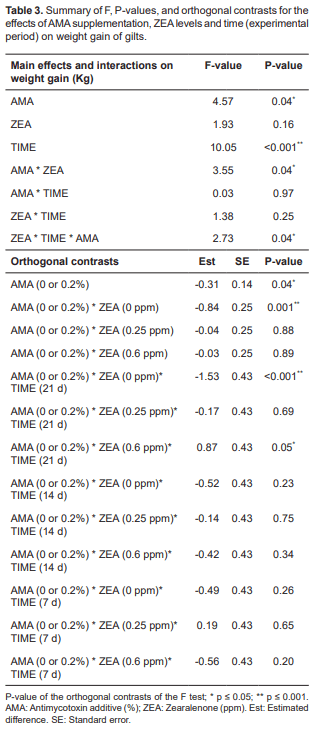
The results also showed significant differences in weight gain (Table 3). Regardless of AMA level (p = 0.04), interaction of weight gain and ZEA (p = 0.16), as well as the combined interaction between weight gain, ZEA and time was not significant (p = 0.25). Time was significant for weight gain (p ≤ 0.001). According to the orthogonal contrasts, the use of AMA is associated with lower weight gain regardless of any other variable (difference of -0.31), and in the absence of ZEA contamination AMA is associated with lower weight gains (p ≤ 0.001). When time and ZEA were taken into consideration, AMA was associated with higher weight gain (difference of 0.87) in the course of the 21 d when ZEA was administered at a level of 0.6 ppm (p ≤ 0.05) and lower body weight (difference of -1.53) in the absence of ZEA supplementation (p ≤ 0.001).
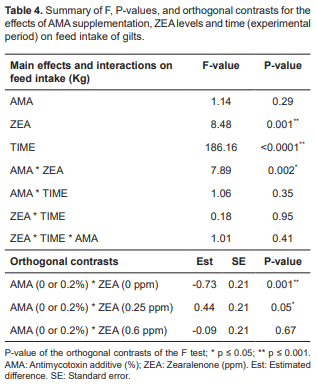
Regarding feed intake (Table 4), AMA alone showed no statistical significance, however, ZEA alone showed significance (p = 0.001), regardless of time and AMA. There was also an effect of time on feed intake (p<0.0001). The interaction of AMA with ZEA levels were significant (p = 0.002). According to the orthogonal contrasts, feed intake was higher when no AMA and nor ZEA were administered (T01; p = 0.001), but also significant when there was presence of AMA at 0.2% and ZEA at 0.25 ppm (p = 0.05).
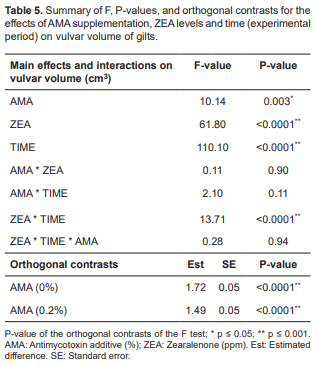
With respect to vulvar volume (cm3), the use of AMA showed difference regardless of ZEA levels (p = 0.003). ZEA levels (p<0.0001), time effect (p<0.0001) and the interaction between ZEA and time (p<0.0001), all showed differences. On the other hand, no differences were observed for the interaction between AMA and ZEA, suggesting that AMA and ZEA have fixed effects on vulvar volume (Table 5). Nevertheless, orthogonal contrasts show that in the absence of AMA (p<0.0001), vulvar volume was much higher (difference of 0.23), with an estimated mean of 1.72 in the absence of AMA and 1.49 in the presence of AMA (0.2%) supplementation, according to the orthogonal contrasts (Table 5).
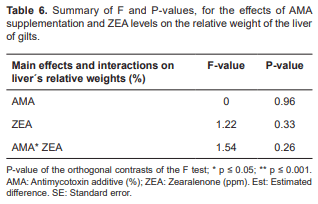
No significant differences were observed in the relative weight of the liver (Table 6).
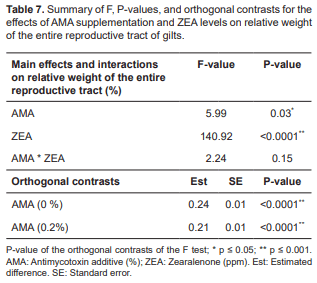
Independently of ZEA levels, the relative weight of the entire reproductive tract was statistically significant in relation to AMA (p = 0.03). A difference was also observed with ZEA in the absence of AMA for the entire reproductive tract (p<0.0001; Table 7). The relative weight of the entire reproductive tract was higher in the absence of AMA (p<0.0001), with an estimated mean of 0.24 in the absence of AMA and an estimated mean of 0.21 in the presence of AMA (0.2%) supplementation, according to the orthogonal contrasts (Table 7).
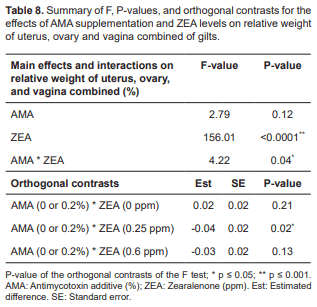
The weight of uterus, ovary and vagina combined was significantly increased only in the gilts that consumed ZEA without AMA supplementation (p<0.0001; Table 8). The interaction between AMA (0.2%) and ZEA (0.25 ppm) caused significant reductions in the weight of uterus, ovary and vagina combined in gilts (Table 8).
Discussion
Our results showed that zearalenone consumption did not affect the live weight of animals. There was a significant difference in time and live weight; this difference resulted because the animals are expected to increase live weight over the course of the study. In addition, the ingestion of feed containing purified ZEA, with no fungus presence, did not alter the nutritional composition of the feed (Hauschild et al., 2007).
In contrast, Teixeira et al. (2011) found no significant difference between weight gained by the control group and the group consuming 0.75 mg/Kg ZEA. It was also reported that low doses of ZEA do not interfere with production efficiency, though it altered some tissues (Gajecka et al., 2016).
The study by Ortiz et al. (2008), who reported that AMA supplementation is beneficial for growth, development and weight gain, was contrasting to our results with respect to feed intake. Our results were corroborated by the study of Andretta et al. (2010) and Teixeira et al. (2011), who reported that ZEA do not significantly affect productivity of the animals.
The clinical signs of hyperestrogenism in the gilts of the present study were noted from the first week. This was consistent with the results of Andretta et al. (2008), who fed 2 mg/Kg ZEA. Thus, an increase in vulvar volume was the first manifestation of ZEA toxicity. Monitoring vulvar volume is an important index for evaluating the extent of ZEA toxicity and the efficacy of AMA.
Supplementation of ZEA and/or AMA did not affect the relative weight of the liver. This finding was also corroborated by Andretta et al. (2008), who found no difference between absolute and relative weight of the liver between the control and the groups that ingested ZEA. However, the liver is responsible for the biotransformation of the mycotoxin (Bryden, 2012). Dumitrescu et al. (2014) reported that ingestion of 0.3 ppm of ZEA by weaned piglets produced liver changes. They reported fibrosis in the perilobular conjunctive septum, interlobular biliary hypertrophy and hypertrophic hepatocytes.
The clinical signs of hyperestrogenism due to ZEA ingestion become more evident with increasing concentrations of the mycotoxin (Doll and Danicke, 2011). The damage to the female reproductive tract attributed to ZEA is due to the competition of mycotoxin and its metabolites with the estrogenic receptors (Bryden, 2012). Ingesting ZEA at concentrations >0.15 mg/Kg results in accumulation of high levels of ZEA in the uterus (Andretta et al., 2008). Oliver et al. (2012) reported similar results for a period of four-weeks in gilts fed diets containing ZEA, after comparing the weight of the entire reproductive tract between the group consuming ZEA and those fed diets without ZEA. Because ZEA and its metabolites have high affinity for estrogen receptors in uterus (Bryden, 2012) they stimulate protein production by the uterine wall causing an increase in uterus weight (Mostrom, 2012).
The effect of AMA is dependent on the presence of ZEA, although none of the parameters measured (weight of uterus, ovary and vagina combined) exhibited fixed effects. In addition, when orthogonal contrasts were applied AMA was evidently responsible for the reduction in the weight of uterus, ovary and vagina combined comparing to T02 and T06 groups.
The use of S. cerevisiae yeast cell walls to enhance feed in animal production has become an increasingly popular method to mitigate the effects of mycotoxins already present in the feed. The addition of S. cerevisiae cell walls in the diet leads to about 80% of ZEA adsorption (Fink-Gremmels and Malekinejad, 2007). The β-glucans present in the cell walls may be responsible for most of the adsorption, with the binding occurring on the YCW surface (Shetty and Jespersen, 2006).
The present study demonstrated that supplementing feeds with AMA have no adverse effect on any of the parameters under investigation. Some parameters were significantly improved, with increased ability to bind/adsorb the different levels of ZEA. Huwig et al. (2001) also reported that the cell wall of some strain of S. cerevisiae were able to adsorb ZEA at a much higher concentration (2.7 ppm) than that of the present study. These results reinforce our hypothesis that the beneficial properties of microorganisms are strain-dependent and emphasize the need for this type of study in new AMA-based products. Our results show the ability of commercial AMA in mitigating the toxic effects of ZEA and the improvement of the parameters used as indices for measuring the level of toxicity to the reproductive organs. However, no particular effect was constant when adding antimycotoxin as they remained the same at both ZEA levels (0.25 and 0.6 ppm).
Acknowledgements
The authors would like to thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Pró-Reitoria de Pesquisa da Universidade Federal de Minas Gerais (PRPq-UFMG) and Lesaffre do Brasil Produtos Alimentícios Ltda.
Conflicts of interest
The authors declare they have no conflicts of interest with regard to the work presented in this report.
This article was originally published in Revista Colombiana de Ciencias Pecuarias 2017; 30:219-299-307. http://dx.doi.org/10.17533/udea.rccp.v30n4a05. This is an Open Access article published under a Creative Commons Attribution License. 







.jpg&w=3840&q=75)