Efficacy of DL-methionine in amelioration of aflatoxicosis in coloured broiler chicken
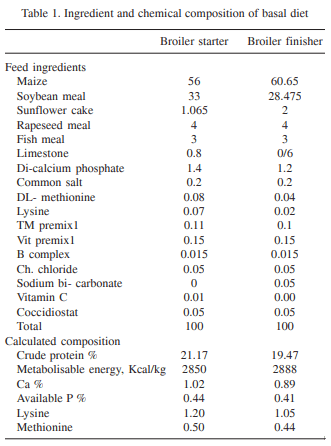
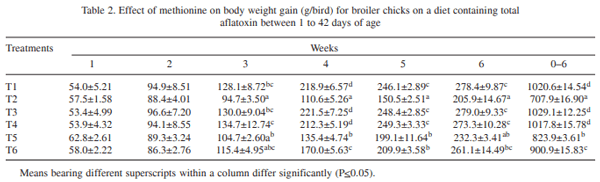
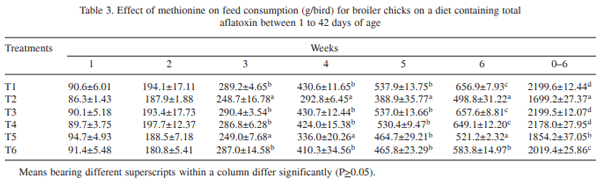
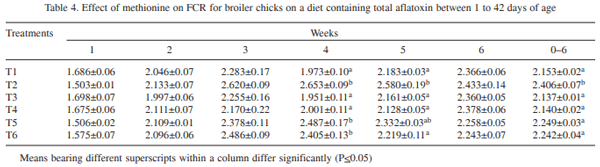
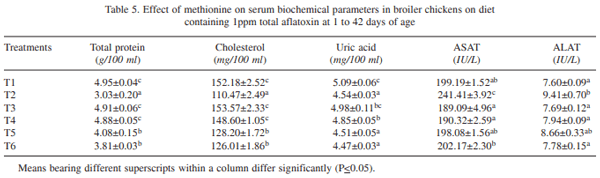
The effect of supplementation of DL- methionine (0.025 and 0.05%) in diet containing 1 ppm total aflatoxin (AF: 76.45% AFB1, 10.52% AFB2, 9.89% AFG1 and 3.14% AFG2) in broiler chickens (day-old to 42 days of age) was investigated. Day-old broiler chicks (180) were divided into 6 treatment groups (T1 – control; T2 – T1 + 1ppm AF, T3 – T1 + 0.025% Met, T4 _ T1 + 0.05% Met, T5 – T2 + 0.025% Met, and T6 – T2 + 0.05% Met). Each diet was fed to 3 replicated groups of 10 birds each from day-old to 42 days of age. The results showed that the body weight gain (BWG) of aflatoxin fed group (T2) was lower than that of control group (T1). The weight gain in groups T3 and T4 did not differ from control, while in groups T5 and T6 gain was higher than that of T2, but lower than that of control. The feed intake (FI) in T5 and T6 was higher than that of T2, but lower than that of control, while FI did not differ in T3 and T4 from that of control. The FCR also increased to 2.41 in T2 in comparison to control (2.15). Barring T2, the FCR in other groups were equal to that of control. Significantly reduced serum protein and cholesterol were reported in T2 compared to T1. The serum protein and cholesterol content in group T5 and T6 remained higher than that of T2, but lower than that of control. ASAT and ALAT activities were increased by feeding aflatoxin (T2) compared to T1. Methionine supplementation to toxin contaminated diet ( T5 and T6) reversed these parameters more or less comparable to that of control. Significant increase in the relative weights of liver and gizzard due to aflatoxin feeding (T2) was reported compared to control. The relative weights of liver and gizzard in groups T5 and T6 were similar to that of control. The relative weight of bursa of Fabricius in T2 was lower compared to control. The relative weights of bursa in groups T5 and T6 was similar to that of control. It was concluded that inclusion of slightly higher methionine (0.025 or 0.05%) in feed of coloured broiler chickens was beneficial to counteract the adverse effects of aflatoxin partially.
Key words: Aflatoxicosis, Broiler, Feed, Methionine.
Ahamad D B. 2000. ‘Pathology of citrinin mycotoxicosis in broiler chicken.’ M V Sc Thesis. Tamil Nadu Veterinary and Animal Sciences University. Chennai, Tamil Nadu, India.
Azzam A H and Gabal M A. 1997. Interaction of aflatoxin in the feed and immunization against selected infectious diseases: Infectious Bursal Disease. Avian Pathology 26: 317– 25.
Bailey R H, Kubena L F, Harvey R B, Buckley S A and Rottinghaus G E. 1998. Efficacy of various inorganic sorbents to reduce the toxicity of aflatoxin and T-2 toxin in broiler chicks. Poultry Science 77: 1623–30.
Basmacioglu H, Oguz H, Col M, Ergul R and Birdabe Y O. 2005. Effect of dietary esterified glucomannan on performance, serum biochemistry and heamatology in broilers exposed to aflatoxin. Czech Journal of Animal Science 50: 31–39.
Busby W F and Wogan G N. 1981. Aflatoxins. Mycotoxins and NNitrosocompounds, Environmental Risks. Vol. 2. Pp. 3–27. (Ed.) R C Shank. CRC Press Inc., Boca Raton, FL.
Chung T K, Erdman J W and Baker D H. 1990. Hydrated sodium calcium aluminosilicate: effects on zinc, manganese, vitamin A, and riboflavin utilization. Poultry Science 69: 1364– 70.
Davidson J N, Babish J G, Delaney K A, Taylor D R and Phillips T D. 1987. Hydrated sodium calcium aluminosilicate decreases the bioavailability of aflatoxin in the chicken. Poultry Science 66: 89.
Devegowda G, Raju M V N L, Afzali N and Swamy V L N. 2000. Mycotoxin picture worldwide: Novel solutions for their counteraction. Passport to the year 2000. Alltech’s 12th Annual Asian Pacific Lecture Tour. Pp. 41–53.
Diaz D E, Hagler W M, Blackwelder J T, Eve J A, Hopkins B A and Andersen K L. 2004. Aflatoxin binders 2. Reduction of aflatoxin M1 in milk by sequestering agents of cows consuming aflatoxin in feed. Mycopathologia 157: 233–41.
Kececi T, Oguz H, Kurtoglu V and Demet O. 1998. Effects of polyvinylpolypyrrolidone, synthetic zeolite and bentonite on serum biochemical and haematological characters of broiler chickens during aflatoxicosis. British Poultry Science 39: 452– 58.
Kubena L F, Harvey R B, Phillips T D, Corrier D E and Huff W E. 1990. Diminution of aflatoxicosis in growing chickens by dietary addition of a hydrated sodium calcium aluminosilicate. Poultry Science 69: 727–35.
Kubena L F, Harvey R B, Huff W E, Elissalde M H, Yersin A G, Phillips T D and Rottinghaus G E. 1993. Efficacy of a hydrated sodium calcium aluminosilicate to reduce the toxicity of aflatoxin and diacetoxyscirpenol. Poultry Science 72: 51–59.
Kubena L F, Harvey R B, Bailey R H, Buckley S A and Rottinghaus G E. 1998. Effects of hydrated sodium calcium aluminosilicate (T-Bind TM) on mycotoxicosis in young broiler chickens. Poultry Science 77: 1502–09.
Ledoux D R, Rottinghaus G E, Bermudez A J and Alonso-Debolt M. 1999. Efficacy of a hydrated calcium aluminosilicate to ameliorate the toxic effects of aflatoxin in broiler chicks. Poultry Science 78: 204–10.
Miazzo R, Rosa C A R, Dequeiroz-Carvalho E C, Mangoli C, Chiacchiera S M, Palacio G, Saenz M, Kikot A, Basaldella E and Dalcero A. 2000. Efficacy of synthetic of synthetic zeolite to reduce the toxicity of aflatoxin in broiler chicks. Poultry Science 79: 1–6.
Naveenkumar B, Reddy K S, Kalakumar B and Reddy A G. 2007. Evaluation of experimental aflatoxicosis and its amelioration by chromium and methionine on performance of broilers. Toxicology International 14: 133–36.
NRC. 1994. Nutrient Requirements of Poultry. 9th revised edn. National Academy Press. Washington D C.
Pons W A, Cucullu A F, Lee L S, Robertson J A, Franz A O and Goldbatt L A. 1966. Determination of aflatoxins in agricultural products: Use of aqueous acetone for extraction. Journal of the Association of Official Analytical Chemists 45: 694–99.
Raju M V L N and Devegowda G. 2000. Influence of esterified glucomannan on performance and organ morphology, serum biochemistry and hematology in broilers exposed to individual and combined mycotoxicosis (aflatoxin, ochratoxin and T-2 toxin). British Poultry Science 41: 640–50.
Rosa C A R, Miazzo R, Magnoli C, Salvano M, Chiacchiera S M, Ferrero S, Saenz M, Carvalho E C Q and Dalcero A. 2001. Evaluation of the efficacy of bentonite from the south of Argentina to ameliorate the toxic effects of aflatoxin in broilers. Poultry Science 80: 139–44.
Santurio J M, Mallmann C A, Rosa A P, Appel G, Heer A, Dageforde S and Bottcher M. 1999. Effect of sodium bentonite on the performance and blood variables of broiler chickens intoxicated with aflatoxins. British Poultry Science 40: 115–19.
Sapocota D, Islam R and Baruah K K. 2007. Protective efficacy of dietary methionine in experimental aflatoxicosis in broilers. Indian Journal of Animal Science 77: 1170–72.
Scheideler S. 1993. Effects of various types of aluminosilicates and aflatoxin B1 on aflatoxin toxicity, chick performance, and mineral status. Poultry Science 72: 282–88.
Shetty P H and Jespersen L. 2006. Saccharomyces cerevisiae and lactic acid bacteria as potential mycotoxin decontamination agents. Trends in Food Science and Technology 17: 48–55.
Shotwell O L, Hesseltine C V, Stubblefield R D and Sorenson W G. 1966. Production of aflatoxin on rice. Applied Microbiology 14: 425–29.
Silambarsan S. 2011. ‘Efficacy of diatomaceous earth, sodium bentonite and zeolite as aflatoxin adsorbents in broiler chickens.’ M. V. Sc. Thesis. IVRI, Izatnagar.
Silambarasan S, Singh R and Mandal A B. 2013. Evaluation of the ability of adsorbents to ameliorate the adverse effects of aflatoxin B1 in broiler chickens. Indian Journal of Animal Sciences 83: 73–77.
Thakur C, Kumar B K, Reddy A G, Reddy A R and Reddy Y N. 2008. Antioxidant and immunomodulatory effect of chlorophyllin on induced aflatoxicosis in broilers. Indian Journal of Animal Sciences 78: 696–99.
Thapa N K. 2008. ‘Pathological effects of aflatoxicosis in layer chicken with special emphasis on reproductive pathology.’ M V Sc Thesis. Tamil Nadu Veterinary and Animal Sciences University.
Veltmann J R Jr, Wyatt R D and Voight M N. 1981. The effect of varying the total sulfur amino acid content in the diets of chicks with aflatoxicosis. Poultry Science 60: 1748 (Abstr.).
Veltmann J R Jr, Wyatt R D Voight M N and Shamsuddin Z. 1983. Influence of dietary sulfur amino acid levels on performance, free amino acids and biochemical parameters in plasma and hepatic glutathione of broiler chicks fed aflatoxin. Poultry Science 62:1518 (Abstr.).
Xue C Y, Wang G H, Chen F, Zhang X B and Cao Y C. 2010. Immunopathological Effects of Ochratoxin A and T-2 Toxin Combination on Broilers. Poultry Science 89: 1162–66.
Inclusion of sulphur containing amino acids to aflatoxin-contaminated feed improved performance in chickens (Veltmann et al. 1981, 1983). Addition of dietary methionine 30-40% above the requirement is reported to alleviate the adverse effects of aflatoxicosis in broiler chickens (Devegowda et al. 2000). However, such a level of methionine was very high and it might not be economical to add that amount. Moreover, methionine is considered to be a toxic amino acid. Therefore, the present study was conducted to determine the efficacy of additional methionine at lower level (excess by 5% and 10%) to ameliorate the toxic effects of aflatoxins. This piece of work was conducted in my laboratory and is the original research work undertaken in the project "Management of Mycotoxicosis in Poultry".
Dr. Ram Singh Bibyan.