Efficacy of fumaric and citric acids in preventing biosynthesis of aflatoxins in poultry feed with variable moisture content
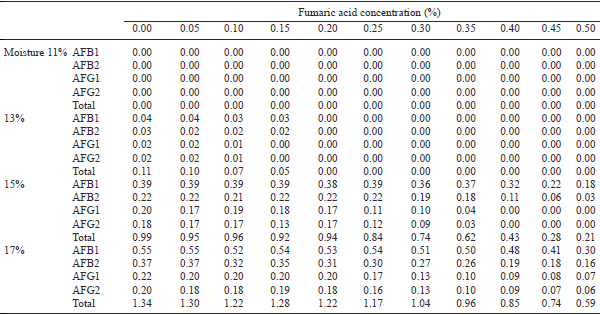
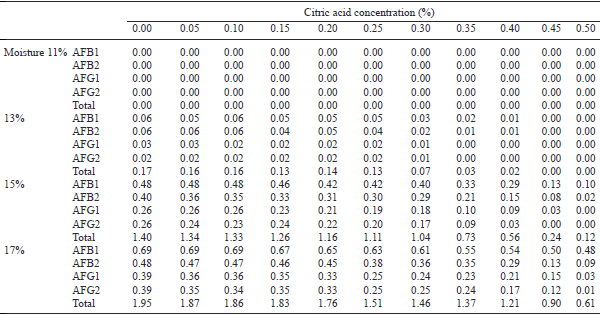
A poultry feed was prepared using conventional feed ingredients free from aflatoxins. The moisture content of the feed was adjusted at 11, 13, 15 and 17%. The feeds with each level of moisture were then mixed with fumaric or citric acid each at various concentrations of 0.00, 0.05, 0.10, 0.15, 0.20, 0.25, 0.30, 0.35, 0.40, 0.45 and 0.50%. Sample (100 g) from each preparation was taken in duplicate, inoculated with fresh spores of aflatoxins producing mould (Aspergillus parasiticus NRRL 2999), incubated at room temperature for 1 month and then analysed for the presence of aflatoxins (AFB1, AFB2, AFG1 and AFG2). The results showed that at 11% moisture level, none of the 4 aflatoxins were recorded in any of the treatments. However, with the increase in moisture in feed from 11 to 17%, oproduction of aflatoxins increased. The concentrations of AFB1 were 40–60 ppb and total AF 110–170 ppb even at 13% moisture level after 1 month storage of such feed. The biosynthesis of any of the aflatoxins was completely inhibited with 0.20% fumaric acid or 0.45% citric acid in feed containing 13% moisture. However, fumaric or citric acid at 0.50% concentration, failed to completely inhibit synthesis of any of the 4 fractions of aflatoxins in feeds containing 15 and 17% moisture level, though with increased concentrations of acids, biosynthesis of total as well as individual fractions of aflatoxins decreased. It is concluded that storage of feed for 1 month with 13% moisture was not safe. Moreover, the production of AF at 13% moisture level can be completely inhibited by adding fumaric acid @ 0.20% or citric acid @ 0.45%. However, at 15 and 17% moisture level in feed, more than 0.50% of fumaric acid or citric acid is required for complete inhibition of biosynthesis of aflatoxins.
Key words: Aflatoxin, Citric acid, Fumaric acid, Poultry feed
Afzal M and Saleem Z. 2004. Effects of addition of a mycotoxin detoxifier in poultry feed containing different levels of aflatoxins on the performance of broilers. Asian-Australian Journal of Animal Science 17: 990–94.
Bartov I. 1982. The nutritional value of mouldy grains for broiler chicks. Poultry Science 61: 2247–54.
Bartov I. 1983. Effects of propionic acid and copper sulphate on the nutritional value of diets containing moldy corn for broiler chicks. Poultry Science 62: 2195–2200.
Bartov I. 1985. Comparative effects of antifungal compounds on the nutritional value of diets containing moldy corn for broiler chicks. Poultry Science 64: 1236–38.
Basmacioglu H, Oguz H, Ergul M Col R and Birdane Y O. 2005. Effect of dietary esterified glucomanna on performance, serum biochemistry and haematology in broiler exposed to aflatoxin. Czech Journal of Animal Science 50: 31–39.
Coulombe Jr R A, Guarisco J A, Klein P J and Hall J O. 2005. Chemoprevention of aflatoxicosis in poultry by dietary butylated hydroxytoluene. Animal Feed Science and Technology 121: 217–25.
Denli M and Okan F. 2006. Efficacy of different adsorbents in reducing the toxic effects of aflatoxin B1 in broiler diets. South African Journal of Animal Science 36: 222–28.
Ghosh M K, Chhabra A, Atreja P P and Chopra R C. 1996. Effect of treating with propionic acid, sodium bisulfate and sodium hydroxide on the biosynthesis of aflatoxin on groundnut cake. Animal Feed Science Technology 60: 43–49.
Higgins C and Brinkhaus F. 1999. Efficacy of several organic acids against molds. Journal of Applied Poultry Research 8: 480–87.
Iheshiulor O O M, Esonu B O, Chuwuka O K, Omede A A, Okoli I C and Ogbuewu I P. 2011. Effects of mycotoxins in animal nutrition: A review. Asian Journal of Animal Sciences 5: 19– 33.
Jones F T. 2005. Control of toxic substances. Feedstuffs, 14 September, 2005.
Leeson S, Diaz G J and Summers J D. 1995. Poultry Metabolic Disorders and Mycotxins. University Books, Guelph, Ontario, Canada. Pp. 249–98.
Lopez L C and Christensen C M. 1976. Effect of moisture content and temperature on invasion of stored corn by Aspergillus flavus. Phytopathology 57: 588–91.
Miazzo R, Rosa C A, De queiroz Carvalho E C, Magnoli C, Chiacchiera S M, Palacio G, Saenz M, Kikot A, Basaldella E and Dalcero A. 2000. Efficacy of synthetic zeolite to reduce the toxicity of aflatoxin in broiler chicks. Poultry Science 79: 1–6.
Oguz H, Kececi T, Birdane, Y O, Onder F and Kurtoglu V. 2000. Effect of clinoptilolite on serum biochrmical and haematological characters of broiler chickens during experimental aflatoxicosis. Research in Veterinary Science 69: 89–93.
Oguz H, Hadimli H H, Kurtoglu V and Erganis O. 2003. Evaluation of humoral immunity of broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Revue de Medicine Veterinaire 154: 483–86.
Ortatatli M and Oguz H. 2001. Ameliorative effects of dietary clinoptilolite on pathological changes in broiler chickens during aflatoxicosis. Research in Veterinary Science 71: 59– 66.
Pons W A, Cucullu A F, Lee L S, Robertson J A, Franz A O and Goldbatt L A. 1966. Determination of aflatoxins in agricultural products: Use of aqueous acetone for extraction. Journal of the Association of Official Analytical Chemists 45: 694–99.
Salunkhe D K, Adsule B N and Padule D N. 1987. Aflatoxins in Foods and Feeds. Text Book, 1st edn.
Santurio J M. 1995. Antifungicos de adsorventes de aflatoxinas em graos: quando usa-los. Anais do simposio Internacional Sobre Micotoxinas e Micotoxicoses em Aves 97– 108.
Sauer F and Burroughs R. 1974. Efficiency of various chemicals as grain mold inhibitors. Transactions of the American Society of Agricultural Engineers 17: 557–59.
Sherwood R F and Peberdy J F. 1974. Production of the mycotoxin zearalenone by fusarium graminearum growing on stored grains. 2. Treatment of wheat grains with organic acids. Journal of the Science of Food and Agriculture 25: 1089–93.
Smith J E and Ross K. 1991. The toxigenic Aspergilli. Smith J E and Handerson R S.
Sur E and Celik I. 2003. Effects of aflatoxin B1 on the development of the bursa of Fabricius and blood lymphocyte acid phosphatase of the chicken. British Poultry Science 44: 558– 66.
Tsai W Y J, Shaoo K P P and Bullarman L B. 1984. Effects of sorbage and propionate on growth and aflatoxin production of sublethality injured Aspergillus parasiticus. Journal of Food Science 49: 8
Aspergillus spp. are primarily storage fungi and are found virtually everywhere in the world that produce aflatoxins (B1, B2, G1 and G2) in feeds. Therefore, achieving complete inhibition of aflatoxin-producing fungus during storage of feed is of utmost importance. This study was conducted with the aim of establishing the efficacy of fumaric and citric acids as mould growth inhibitor in poultry feed.
Dr. Ram Singh Bibyan.
Dear Colleagues,
Thanks for your article, that's very interesting for my work.
This month, I begin to test an additive with citric acid and some natural antioxidants, in a chickens farm (in vivo test).
If my results are positive, what do you think, could we try a collaboration, in a joint project or a private research?
My e-mail address: calbulescu04@gmail.com
Best regards,
PhD Carmen ALBULESCU, AMD INITIATIVE, Bucharest, ROMANIA

How to make storage low moisture and avoid moisture loss?