INTRODUCTION
Mycotoxins are considered important contaminants of maize-based products (Abdallah et al. 2015). The natural occurrence of aflatoxins, fumonisins, zearalenone, ochratoxin A, trichothecenes, patulin and penicillic acid is often observed in animal feed, and include the concomitant occurrence of two or more mycotoxins. These natural contaminants of cereals are found worldwide mainly in corn and its derived products.
Pigs are highly susceptible to aflatoxins and fumonisins, especially in the weaning stage where they may cause a variety of chronic or acute syndromes, depending on the level of consumption (Döll et al. 2003, Oswald et al. 2005). Studies have shown contamination of piglet feed with aflatoxins and fumonisins often results in poor performance, decrease in voluntary feed intake, weight loss and suppressed immune function. Other findings, including effects on the liver, alterations of serum biochemical parameters and gastrointestinal lesions, have also been reported (Dilkin et al. 2003).
Fumonisin toxicosis in swine is characterized by injury to the pulmonary, hepatic, cardiovascular and immune systems as well as early and widespread alterations of sphingolipid metabolism, growth rate and carcass composition (Haschek et al. 2001). As a consequence of the importance of these two mycotoxins for swine, the co-contamination of experimental diets with aflatoxins and fumonisins is important to simulate field situations.
Interestingly, the cholinergic system responds to various insults including oxidative stress, an important event that has been related to aflatoxicosis (Pohanka 2014). Acetylcholinesterase (AChE; EC 3.1.1.7) and butyrylcholinesterase (BuChE; EC 3.1.1.8) are two classes of cholinesterase enzymes involved incontrol of learning, memory, cortical organization of movement, immune responses, hydrolysis of esters of choline and xenobiotics (Soreq and Seidman 2001, Anglister et al. 2008). Furthermore, studies have also demonstrated that mycotoxins can affect cholinergic enzymes suggesting that the higher the enzymatic activity the lower the cellular oxidative stress (Lautert et al. 2014).
Despite improvements in terms of detoxification and decontamination of animal feed, other strategies have been developed to supplement diets (Ferreira et al. 2009). Among the different processing methods, the spray-dried porcine plasma (SDPP) has been shown to be a good source of protein in the piglet diet (Butolo et al. 1999, Weaver et al. 2014). SDPP addition improves the digestibility and palatability of diets and has important nutritional characteristics due to the presence of immunoglobulins, low molecular weight proteins responsible for the immune response in the first two weeks of piglet life (Campbell et al. 2008, Gattás et al. 2008). Previous studies have shown that SDPP improves growth performance and reduces the inflammatory state when SDPP was used in piglet’s diet (Pierce et al. 2005, Schuh et al. 2016). Importantly, SDPP use can be associated with minimum risk of pathogen transmission (Pujols et al. 2008, 2014) and the inclusion of 6% SDPP in the diets is economically viable for piglets (Müller et al. 2018a).
Kenney and Ganta (2014) demonstrated the interaction between nervous and immune systems. According to these authors, the central autonomic system is informed of peripheral immune status via several pathways, such as the production of cytokines. Cytokines alter the activity of sympathetic and parasympathetic nerves in lymphatic organs, and these alterations lead to a modulation of peripheral immune response by cholinergic anti-inflammatory pathway in immune cells. To our knowledge, there are no reports of the effects of SDPP on cholinergic pathway. Conversely, a few studies indicate the potential use of SDPP in the improvement of immune system (Nofrarías et al. 2007), including during mycotoxin intoxication (Müller et al. 2018b). Moreover, the antiinflammatory mechanism of action of SDPP was recently associated with reduction of lymphocyte activation (Pérez-Bosque et al. 2016), an immune cell that express cholinergic receptors in the plasma membrane. Thus, the aims of this study were to evaluate the effect of SDPP supplementation on cholinesterase enzymes and its relationship with animal behavior of weaning piglets exposed to diets contaminated with mycotoxins.
MATERIALS AND METHODS
ETHICS COMMITTEE
This experiment was approved by the Ethics Committee on Animal Research of University of Santa Catarina (UDESC), under protocol number 01.34.15.
SPRAY-DRIED PORCINE PLASMA
SDPP (AP 920®) was purchased from APC do Brasil Ltda (Campinas, São Paulo, Brazil). According to the manufacture, AP 920 should be added in the concentrations of 6 to 5%, 3 to 2.5% and 1.5 to 1% in the initial, middle and final growth phase of piglets, respectively. In our study, based on the manufacturer’s recommendation and on a previous published study (Weaver et al. 2014), we chose to use the concentration of 6% SDPP in the diets, given the mycotoxin challenge.
MYCOTOXIN PRODUCTION
The mycotoxin production method used in this study was previously detailed by Müller et al. (2017). Aflatoxins were produced by fermentation in converted rice under constant stirring and controlled temperature. The NRLL 2999 strain of Aspergillus parasiticus was used according to the method described by West et al. (1973). Ground rice was added to the feed as required, never exceeding 1% of the total diet. The autoclaved corn was inoculated with a Fusarium verticillioides strain and allowed to incubate at 25 °C for 30 days. Thereafter, the corn was dried at 65 °C for 48 h, milled and analyzed for fumonisins content.
Knowing the total levels of mycotoxins produced, i.e., 130 mg of aflatoxins/kg of rice and 110 mg of fumonisins/kg of corn. It was calculated the inclusion rate of contaminated material on the diet aiming to formulate the two experimental diets (MYC and MYC+SDPP) with desired levels of contamination, 300 µg/kg of aflatoxins and 8.000 µg/kg fumonisins.
QUANTIFICATION OF MYCOTOXINS IN DIETS
The levels of aflatoxins and fumonisins in all diets were measured using high performance liquid chromatography technique, with imuno affinity purification, post column derivatization and fluorescence detection. The extraction and purification procedures was performed according to Aflatest WB and Fumonitest WB columns guide (VICAM Science Technology, Watertown, MA, USA). Post column derivatization for aflatoxins was obtained by a photo chemical reactor PHRED (Aura Industries Inc., NY, USA) and for fumonisins was used a chemical reaction procedure according to EN 16006 (European Committee for Standardization 2011). The methods presented a limit of quantification (LQ) of 0.5 µg/kgfor each aflatoxins (B1, B2, G1 and G2) and 100 µg/kg for fumonisin B1 and B2, respectively. Analysis of this diet showed that there was not a natural detectable contamination for aflatoxins (levels < 0.5 µg/kg), but for fumonisin (B1 and B2) it was from 140 to 180 µg/kg (diets CTL and SDPP, respectively). The final level of contamination observed in the artificially contaminated diets (MYC and MYC+SDPP) were shown in Table I.
ANIMALS
Twenty-four castrated male piglets, from a commercial line, weighing 7.15± 0.61 kg (24 ± 2 days) were used. The piglets were housed in pairs in 1.2 x 0.5 m metal pens, with slatted polypropylene floor and with feed and water ad libitum. The environment was heated by automatic electric heaters (23±2 °C). The experiment was carried out in teaching facilities in the municipality of Chapecó, South of Brazil.
TREATMENTS
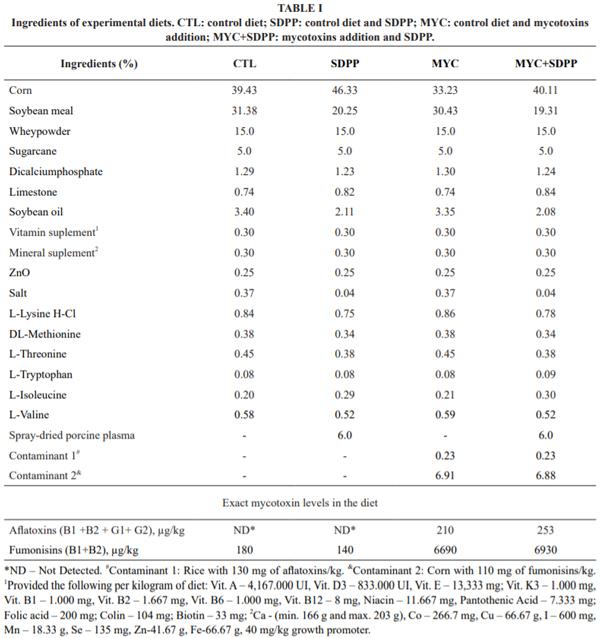
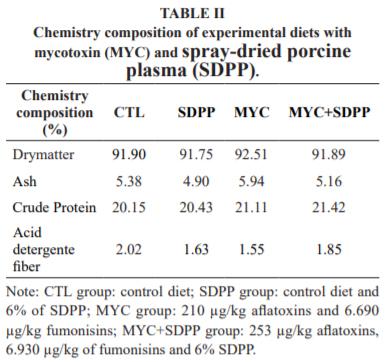
Four isonutritive diets were formulated according Rostagno et al. (2011) (i.e. all with calculated composition of 0.85% calcium, 0.43 phosphorus available, 0.28% sodium, 3.14 Mcal/kg metabolible energy, 21% crude protein, 1.65% lysine digestible, 0.91% methionine + Cis digestible, 0.30% tryptophan digestible, 1.11% threonine digestible, 1.37% valine digestible, and 0.91% isoleucine digestible), with corn and soy as the main ingredients of the diet (Table I). The chemical composition of the diets was made according to the recommended methodologies by Brasil (2005) and results showed in Table II.
The four diets represent the four treatments provided to the groups of post weaning animals, differing in contamination with aflatoxins and fumonisins in the addition or not of 6% of SDPP during 15 days of experiment. The animals were divided in four groups: CTL group, piglets fed with a control diet (without experimental contamination); SDPP group, piglets fed a control diet and SDPP 6%; MYC group, piglets fed an experimental diet containing 210 µg/kg aflatoxins and 6.690 µg/ kg fumonisins; and MYC+SDPP group, piglets fed an experimental diet containing 253 µg/kg of aflatoxins, 6.930 µg/kg fumonisins and 6% SDPP. The concentrations of aflatoxins and fumonisins used in this study were based on a study conducted by Drabek et al. (1989) and Weaver et al. (2014), respectively, that concluded that this concentration reduces the performance of pigs and the biological value of their meat. The concentration of SDPP was based on a study conducted by Weaver et al. (2014).
SAMPLE COLLECTION AND PREPARATION
Blood was collected at days 5, 10 and 15 of experiment by vena cava puncture in vaccutainer tubes using EDTA as anticoagulant. The samples were hemolyzed with phosphate buffer, pH 7.4, and containing 0.03% Triton X 100 and stored at -20 °C for one week until AChE analysis. Serum was obtained from whole blood collected in tubes without anticoagulant and centrifuged at 3.000 rpm for 15 min. Samples were stored at -20 °C until use.
At day 15 of experiment, five animals per group were euthanized. Fragments of liver, intestine (duodenum, jejunum and ileum) and brain were removed and kept in 10% formalin buffer for histopathological analysis. Cerebral cortex and cerebellum were segregated and placed in a solution of 10 mM Tris–HCl, pH 7.4 on ice. Brain structures were homogenized in a glass potter in Tris–HCl solution. Aliquots of resulting brain structure homogenates were stored at -20 °C until use. Protein was determined previously in a strip that varied for each structure: cerebral cortex (2 mg/ mL) and cerebellum (2 mg/mL) as determined by the Coomassie blue method according to Bradford (1976), using bovine serum albumin as standard solution.
ACETYLCHOLINESTERASE ACTIVITY
Cerebral AChE activity was determined by Rocha et al. (1993), with modification. The reaction mixture (300 µL final volume) contained 100 mM K+-phosphate buffer, pH 7.5 and 1 mM 5.5′-dithiobisnitrobenzoic acid (DTNB). The enzyme (2 mg of protein) was pre-incubated at 25 °C with 0.8 mM acetylthiocholine iodide (AcSCh) as substrate for 2 min. The formation of the 5.5′-dithio-bis-acid-nitrobenzoic anion was measured spectrophotometrically by absorbance at 412 nm. The results were expressed in μmol AcSCh/h/mg of protein.
The specific activity of whole blood AChE was determined according to Worek et al. (1999). AChE activity was calculated from the quotient between AChE activity and hemoglobin content and the results were expressed as mU/μmol of whole blood.
BUTYRYLCHOLINESTERASE
BuChE activity was determined in serum by Ellman et al. (1961), with modification. The reaction mixture (300 µL) contained 100 mM potassium phosphate buffer, pH 7.5, and 1mM DTNB. The method is based on the formation of the yellow anion, 5.5’-dithio-bis-acid nitrobenzoic, measured by absorbance at 412 nm after 2 min incubation at 25 °C. Enzyme activity was expressed in µmol BuSCh/h/mg of protein.
HISTOPATHOLOGY
Sagittal sections (3 mm thick) of the liver, intestine and brain were mounted and fixed in 10% buffered formalin solution. Slides were stained with hematoxylin and eosin (H&E) for histopathological analysis.
BEHAVIORAL PROCEDURE
The behavioral test was carried out in two moments. The first one was based on the use of the 0-1 (zero-one) method adapted from Castro (2010), where observations were made of the postfeeding period on the last day of each stage, i.e., on days 5, 10 and 15 after diet supplementation. Animals had free access to feed, except for the behavioral tests when the ration was offered at 10 different moments of each evaluated day, i.e., for five minutes immediately after feeding (totaling 50 min of observations per day). The first evaluation occurred at 07:00 AM and the last at 07:00 PM of each day (eight other evaluations occurred between these periods). When the expected behavior was visualized (the same observer was used throughout the experiment), it was recorded as “1” and when it was not observed at that time it was recorded as “0” (zero). In the echogram, the behaviors were classified as resting (resting or sleeping), eating or other behavior (playing or walking). These data were later tabulated and transformed to numbers for subsequent statistical analysis. It is important to note that a mean of behaviors of each piglet of the 10 moments of each day was made, and therefore only one data for each animal per day was tabulated for statistical analysis.
The second behavioral analysis was conducted on day 15 of experiment. A new objected was insertedin the pens to determine the behavior of each piglet to a novelty in the environment, following the method adapted by Cramer (2014). Metal chains measuring 25 cm in length were attached to the top of the cage, hanging at the height of the pigs, which allowed the chain to have one side free to be moved according to the interaction of the animal. This interaction was recorded during 30 minutes (10:00 to 10:30 AM) using a digital camera on day 15 of experiment. To distinguish the two animals from each cage, these were identified with nontoxic paint markers in the back. The length of time each animal took to make the first contact with the new object and the length of time that the animal remained interacting with it was registered.
STATISTICAL ANALYSIS
Data were first analyzed by descriptive statistics for contingency of the information and for further assumptions, and were presented as descriptive (mean and standard deviation). The data were tested for normality of variance by the KolmogorovSmirnov test, skewness and homogeneity by the Levene test, and did not show normal distribution. Therefore, data were transformed to logarithm and submitted first to one-way analysis of variance (ANOVA) followed by the Duncan test to verify differences among groups in the three moments separately. Results of serum AChE and BChE activities were submitted to repeated measures one-way ANOVA to analyze if these parameters showed significant differences over time. Groups of the experiment II were compared considering the three evaluated moments (days 5, 10 and 15 of experiment). Data were considered significantly different when P<0.05. The statistical analysis was carried out with R-language, v.3.3.0, 2012 (R Development Core Team).
RESULTS
CLINICAL SIGNS AND HISTOPATHOLOGICAL ANALYSES
No apparent clinical signs were observed in the piglets that received mycotoxins in the diet, suggesting absence of clinical intoxication. No histopathological lesions were observed in the liver, intestine or brain in the piglets of this experiment.
AChE ACTIVITIES
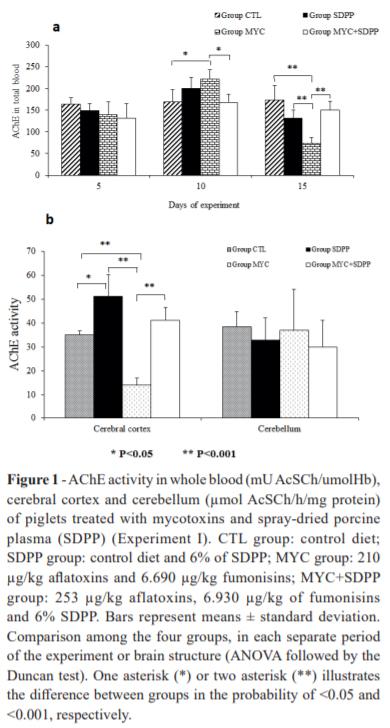
AChE activity in whole blood did not show significant differences among the groups at day 5 of experiment (Fig. 1). At day 10, AChE activity significantly increased in MYC (50%) group when compared to CTL and MYC+SDPP (65%) groups (P<0.05). However, at day 15 of experiment, whole blood AChE activity was significantly decreased in MYC 100% compared to control, 65% compared to SDPP and increase 75% MYC+SDPP compared to MYC group. In cerebral cortex, AChE activity was significantly affected in MYC group when compared to other treatments (CTL (decrease 15%), SDPP (decrease 35%) and MYC+SDPP (increase 25%)). The treatments did not affect AChE activity in cerebellum (P<0.05).
A significant increase on blood AChE activity was observed from day 5 from 10 (P=0.04; P<0.001) in the SDPP and MYC groups. Conversely, a reduction on AChE activity was observed from day 5 from15 (P<0.001) and from day 10 from 15 (P<0.001) in the MYC group.
BChE ACTIVITIES
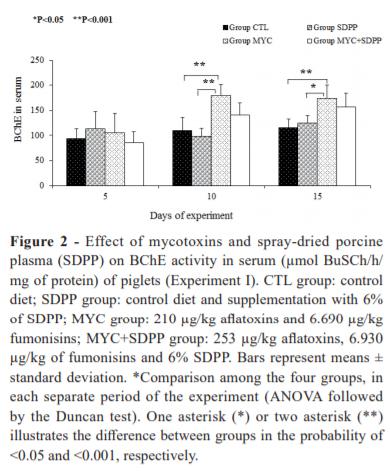
At day 5 of experiment, serum BChE activity did not differ among the groups (Fig. 2). At days 10 and 15 of experiment, BChE activity increased in piglets that received diets containing mycotoxins (MYC group) when compared to control (CTL (100%) and SDPP (75%) at 10 days) (CTL (75%) and SDPP (35%) at 15 days) (P<0.05). The SDPP supplementation associated to mycotoxin (MYC+SDPP group) did not show significant difference when compared to MYC group.
In the MYC group, a significant increase on BChE activity in serum was observed from day 5 from 10 (P=0.02) and from day 5 from 15 (P=0.05). An increase in BChE also was observed from day 5 from 15 of experiment in the MYC+SDPP group.
BEHAVIORAL TESTS
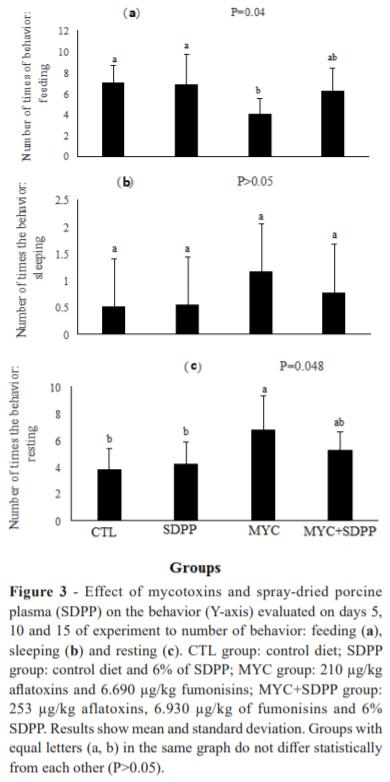
The behavioral results are shown in Fig. 3. The animals that received mycotoxins in the diet (MYC group) spent most of the time sleeping. Moreover, the resting behavior of MYC group was statistically increased when compared to the other groups and, conversely, the feeding behavior was decreased (P<0.05) when compared to CTL and SDPP groups. Animals that received SDPP (MYC+SDPP group) showed similar behavior patterns to animals of CTL and SDPP groups (P>0.05). No statistical difference was observed among the groups in the test of interaction with objects (P>0.05).
In summary, the number of times of active behaviors of MYC group was decreased when compared to the others (CTL group: 15.1, SDPP group: 15.2, MYC group: 11.9, and MYC+SDPP group: 14.5). Nonetheless, the number of times of resting behavior was increased in animals of MYC group (CTL group A: 4.8, SDPP group: 4.7, MYC group: 7.6, and MYC+SDPP group: 5.8).
DISCUSSION
SDPP is an excellent source of proteins for newborn piglets due to great amino acid ratio and high level of globular proteins that stimulate the feed intake and performance during the critical post weaning phase (Cromwell 2006). In the present study, SDPP supplementation showed beneficial effects on behavior of piglets exposed to mycotoxin co-contamination food. In a previous study with these animals published by Muller et al. (2018a) it was observed that the animals that received SDPP had greater weight gain and a lower incidence of diarrhea in a diet with low and high mycotoxin, another positive effect of supplementation.
The ingestion of diets containing mycotoxins resulted in a decrease of feed-intake behavior of animals and an increased frequency of sleeping and resting behaviors. These results are in agreement with Pierce et al. (2005) which verified that mycotoxin intoxication in pigs decrease voluntary feed intake. Due to the limited digestive capacity of piglets, the addition of SDPP in the post weaning increases the locomotion and improves active behavior of animals. Touchette et al. (1996) have associated a stimulatory plasma effect with the ingestion and maintenance of intestinal villi in piglets, which may have occurred due to the action of plasma or to the stimulus it exerts on food consumption.
SDPP supplementation increased AChE activity in cerebral cortex of piglets. The lack of uniformity in the AChE profile may be a reflection of the functional heterogeneity in the central cholinergic system. Cerebellum presents few cholinergic neurons, which is likely to explain the low AChE activity (Das et al. 2001). Our findings indicate that mycotoxins increased whole blood AChE and serum BChE activities of piglets throughout the experiment. This activation leads to fast acetylcholine degradation, as observed at day 15 of experiment, and a subsequent downstimulation of acetylcholine receptors causing undesirable effects on locomotion functions (Soreq and Seidman 2001). Furthermore, based on these results, it is suggested that the mycotoxin-related increase in BChE activity decreases butyrylcholine efficiency in the synaptic cleft and serum, thus contributing to regressive behavior.
The use of SDPP of pig origin has demonstrated better results when compared to that of bovine origin, suggesting a specific effect of immunoglobulin (Dalto et al. 2013). Furthermore, the beneficial effect of SDPP is related to the high levels of IgG that remain active in the manufactured plasma (Pierce et al. 2005, Campbell et al. 2008). AChE is an enzyme present in the central nervous system and lymphocytic membrane that is responsible for regulating acetylcholine, which modulates the activation and differentiation of lymphocytes improving immune response (Wessler and Kirkpatrick 2001).
SDPP supplementation was able to avoid the changes in whole blood AChE activity at days 10 and 15 of exposition to mycotoxins in feed. The data suggest that SDPP can decrease free acetylcholine in order to minimize and/or modulate inflammation caused by aflatoxins and fumonisins throughout the experiment. The increase of AChE in cerebral cortex was observed at the end of the experiment. The addition of SDPP avoided mycotoxin-related changes in BChE activity. Thus, SDPP treatment can be contributing to a greater absorption of immunoglobulins and, consequently to increase the passive immunity of piglets.
The results of this experiment can contribute to the potential production and quality of the animal diet, since the presence of mycotoxin can affect cholinergic system causing less response to food intake, limited locomotion and development of immature piglets. On the other hand, the supplementation with SDPP is an excellent source of proteins for newborns, improving the nutrient recovery, reducing the overstimulation of the immune response, and preserving the use of nutrients for better productive performance.
CONCLUSION
In summary, the present study showed that the co-contamination of aflatoxins and fumonisins to piglet diets avoids the increase and reduction of seric AChE and BChE activities, respectively. Subclinical mycotoxin poisoning in piglets (MYC group) reduces the activity of cerebral AChE, thus interfering in the hydrolysis of the neurotransmitter acetylcholine in the synaptic cleft and, consequently, altering the behavior of the animals. The SDPP supplementation avoids changes in blood and brain AChE activity in animals exposed to mycotoxin co-contaminated diets, directly or indirectly, by unknown mechanisms.
ACKNOWLEDGMENTS
We would like to thank Ajinomoto do Brasil (Nutract Alimentos and Vitamix Nutrição Animal) for supplying some of the ingredients used in the present study. We also thank the people who positively contributed with this project: Jeferson Gugel, William Raphael Lorenzetti and Régis Adriel Zanette.
This article was originally published in Anais da Academia Brasileira de Ciências. vol.91, no.2, Rio de Janeiro,2019Epub, July 01, 2019. https://doi.org/10.1590/0001-3765201920180419. This is an Open Access article distributed under the terms of the Creative Commons Attribution License.