Effect of Supplementation of Toxin Binder (Mycodetox B2) on Liveability, Immune Response and Pathology of Organs During Aflatoxicosis in Japanese Quails
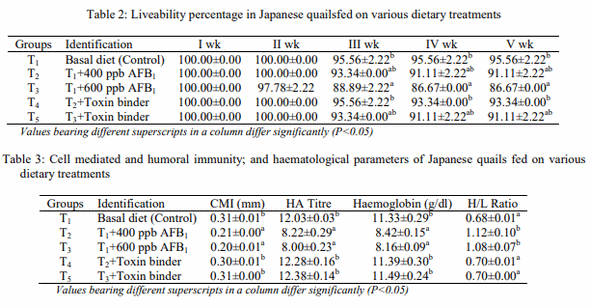
The present study was undertaken to evaluate the efficacy of toxin binder (Mycodetox B2) in ameliorating aflatoxicosis in Japanese quails. The Mycodetox B2 contained sodium bentonite (30.30%), zeolite (30.30%), mannan oligosaccharide (15.15%), methionine (18.94%), butylated hydroxyanisole (3.74%) and Zinc (1.52%). Day-old quail chicks (n=225) were divided into five treatment groups, viz. T1: control (Basal diet); T2: T1+400 ppb Aflatoxin B1 (AFB1); T3: T1+600 ppb AFB1; T4: T2+Toxin binder; T5: T3+Toxin binder. Each diet was fed to three replicated groups of 15 birds each from day-old to 35 days of age. The results showed that overall liveability percentage in T3 was lower (P<0.05) than that of control. The overall liveability percentage in T2 was lower (P<0.05) than that of T4. The overall liveability percentage in T4 and T5 was higher (P<0.05) than that of T3 and statistically similar to that of control. The CMI and HA titre value of T1 was higher (P<0.05) than those of T2 and T3. The CMI and HA titre value in T4 and T5 was higher (P<0.05) than those of T2 and T3 and statistically similar to that of control. The haemoglobin (Hb) value of T1 was higher (P<0.05) than those of T2 and T3. The Hb value in T4 and T5 was higher (P<0.05) than those of T2 and T3; and statistically similar to that of control. The heterophil/lymphocyte (H/L) ratio of T2 and T3 was higher (P<0.05) than that of T1. The H/L ratio of groups T4 and T5 was lower (P<0.05) than those of T2 and T3 and statistically similar to that of control. Grossly, aflatoxicosis in T2 and T3 caused enlargement, paleness, petechial haemorrhage and rounded borders in liver; congestion and a few haemorrhagic spots were also seen in the heart, lungs, small intestine and proventriculus. The organs of T4 and T5 groups were normal as that of T1. Histopathologically, in T2 and T3, marked destruction of hepatic cords, and dilatation and congestion of central veins, adenomatous necrotic foci, fatty change, hepatocytic degeneration and marked infiltration of mononuclear cells around the portal area, hypertrophy of the hepatocytes and hyperplasia of the bile duct with destruction of portal vein were observed in the liver. Intestine showed degenerative changes, severe necrosis and infiltration of inflammatory cells in groups T2 and T3. In groups T4 and T5, the architectural and cellular organization was normal and comparable to that of T1. It was concluded that the inclusion of 400 or 600 ppb dietary AFB1 resulted in reduced liveability, suppression of immunity, decreased haemoglobin concentration, increased H/L ratio; and gross and histopathological alterations in organs of quail chicks. The inclusion of toxin binder (Mycodetox B2) to the AFB1 contaminated diet ameliorated the adverse effects on liveability, immunity, haematological parameters, and gross and histopathology of organs in Japanese quails.
Keywords: Aflatoxin B1, Liveability, Immunity, Organ pathology, Japanese quail, Mycodetox B2.
AOAC (1995). Official methods of analysis. Association of Official Analytical Chemists, Washington DC, USA.
Bakshi CS (1991). Studies on the effect of graded dietary levels of aflatoxin on immunity in commercial broilers. M.V.Sc. Thesis Submitted to Deemed University, IVRI, Izatnagar, India.
Basmacioglu H, Oguz H, Ergul M, Col R and Birdane YO (2005). Effect of dietary esterified glucomannan on performance, serum biochemistry and haematology in broilers exposed to aflatoxin. Czech Journal of Animal Science, 50: 31-39.
Chang CF, Hamilton PB and Weeks BA (1976). Impairment of leukocytechemotaxis and phagocytosis. American Society for Microbiology, 181: 10.
Churchil RR, Praveena PE and Maldhure NA (2014). Effect of esterified glucomannan in amelioration of aflatoxin induced microscopic changes in broiler chicks. Journal of Poultry Science and Technology, 2: 36-37.
Corrier DE and Deloach JR (1990). Evaluation of cell mediated cutaneous basophil, hypersensitivity in young chickens by an interdigital skin test. Poultry Science, 69: 403-408.
Culling CFA (1968). Histological and histochemical staining techniques, (3rd Edn.) Woodsworth Publication Pvt. Ltd., London. DeDuve C and Wattiaux R (1966). Functions of lysosomes. Annual Review of Physiology, 28: 435-492.
Denli M, Blandon JC, Guynot ME, Salado S and Perez JF (2009). Effects of dietary Afladetox on performance, serum biochemistry, histopathological changes and aflatoxin residues in broilers exposed to aflatoxin B1. Poultry Science, 88: 1444-1451.
Deo P, Blaney B and Dingle J (1998). Aflatoxin decreases immunity and stress reaction in poultry. Proceedings on Australian Poultry Science Symposium, 10: 172-175.
Ghosh RC and Chauhan HVS (1991). Suppression of cell mediated immunity by purified aflatoxin B1 in broiler chicks. Indian Journal of Animal Health, 30(1): 23-26.
Ghosh RC, Chauhan HV and Jha GJ (1991). Suppression of cell-mediated immunity by purified aflatoxin B1 in broiler chicks. Veterinary Immunology and Immunopathology, 28: 165-172.
Giambrone JJ, Ewert DL, Wyatt RD and Eidson CS (1978). Effect of aflatoxin on the humoral and cell-mediated immune systems of the chicken. American Journal of Veterinary Research, 39: 305-308.
Giambrone JJ, Diener UL, Davis ND, Panangala VS and Hoerr FJ (1985). Effects of purified aflatoxin on broiler chickens. Poultry Science, 64: 852-858.
Gopi K (2006). Influence of melatonin on aflatoxicosis in broiler chickens. M.V.Sc. Thesis, IVRI, Izatnagar, India. Huff WE, Kubena LF, Harvey RB, Hagler VM, Swanson SP, Phillips TD and Creger CR (1986). Individual and combined effects of aflatoxin and deoxynivalenol (DON, vomitoxin) in broiler chickens. Poultry Science, 65: 1291-1298.
Kadian SK, Monga DP and Goel MC (1988). Effect of aflatoxin B1 on DTH and phagocytic activity of reticuloendothelial system in chickens. Mycopathologica, 104: 33-36.
Katole SB, Kumar P and Patil RD (2013). Environmental pollutants and livestock health: a review. Veterinary Research International, 1(1): 1-13.
Kececi T, Oguz H, Kurtoglu V and Demet O (1998). Effects of polyvynylpoly-pyrrolidone, synthetic zeolite and bentonite on serum biochemical and haematological characters of broiler chickens during aflatoxicosis. British Poultry Science, 39: 452-458.
Khatke PA, Singh R and Mandal AB (2012a). Efficacy of biological adsorbents to ameliorate aflatoxicosis in broiler chicken: Effect on immune response and histopathology of liver. Indian Journal of Poultry Science, 48(1): 27-32.
Khatke PA, Singh R, Mandal AB and Tyagi PK (2012b). Evaluation of the ability of Saccharomyces cerevisiae and mannan oligosaccharides to ameliorate the adverse effects of aflatoxin B1 in broiler chickens. Indian Journal of Poultry Science, 47(2): 176-182.
Khatke PA, Singh R, Mandal AB and Tyagi PK (2012c). Ability of biological adsorbents to ameliorate aflatoxicosis in broiler chicken: Effect on blood biochemicals and organ weights. Indian Journal of Poultry Science, 48(1): 117-121.
Mohanamba T, Rao MR and Habibi SMM (2007). Aflatoxin contamination in animal feed. Indian Veterinary Journal, 84(4): 416-420.
Oguz H, Hadimli HH, Kurtoglu V and Erganis O (2003). Evaluation of humoral immunity of broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Journal of Veterinary Medicine, 154: 483- 486.
Patel VR, Choubey M, Trangadiya BJ and Raval AP (2015). Mycotoxins in feed and their amelioration: a review. International Journal of Animal and Veterinary Sciences, 2: 28-33.
Pathak GP, Sharma R, Patil RD, Sharma DK and Varshneya C (2017). Effect of dietary supplementation of esterified glucomannan against aflatoxin B1 -induced toxicity in broiler chicks. Journal of Poultry Science and Technology, 5(1): 1-6.
Patial V, Asrani RK and Patil RD (2013). Nephrotoxicity of ochratoxin-A in Japanese quail: A clinico-pathological study. Journal of Poultry Science and Technology, 1(1): 07-12.
Patil RD and Degloorkar NM (2016a). Protective effect of Bantox® on ochratoxin A-induced liver Damage in broilers: A histopathological study. Journal of Poultry Science and Technology, 4(4): 46-51.
Patil RD and Degloorkar NM (2016b). Nephrotoxicity of ochratoxin A in broiler chicken and its amelioration with Bantox®: Histopathological assessment. Journal of Poultry Science and Technology, 4(4): 52-58.
Patil RD and Degloorkar NM (2018). Ameliorative efficacy of commercial mycotoxin binder (Bantox®) against ochratoxin A-induced microscopic pathology in broiler birds. Journal of Poultry Science and Technology, 6(2): 26-30.
Patil RD, Degloorkar NM and Pawar PK (2017a). Ameliorating effects of Bantox® on clinical manifestation and growth performance of broiler chicken fed with ochratoxin A. Journal of Poultry Science and Technology, 5(3): 22-27.
Patil RD, Degloorkar NM and Pawar PL (2017b). Effects of ochratoxin A feeding on organ weights and gross pathological changes in broiler chicken and its amelioration with Bantox®. Journal of Poultry Science and Technology, 5(4): 44-51.
Patil RD, Degloorkar NM, Moregaonkar SD and Kulkarni GB (2005). Ameliorative efficacy of Bantox in induced ochratoxicosis in broilers: A haemato-biochemical study. Indian Journal of Veterinary Pathology, 29(2): 90-94.
Patil RD, Dwivedi P and Sharma AK (2006). Critical period and minimum single oral dose of ochratoxin A for inducing developmental toxicity in pregnant Wistar rats. Reproductive Toxicology, 22(4): 679-687.
Patil RD, Sharma R and Asrani RK (2014). Mycotoxicosis and its control in poultry: a review. Journal of Poultry Science and Technology, 2(1): 1-10.
Pons D, Cucullu AP, Lee LS, Robertson JA and Goldblatt LA (1966). Determination of aflatoxins in agricultural products: Use of aqueous acetone for extraction. Journal of Analytical Chemistry, 49: 544-552.
Reddy RA, Reddy VR, Rao PV and Yadagiri B (1982). Effect of experimentally induced aflatoxicosis on the performance of commercial broiler chicks. Indian Journal of Animal Sciences, 52: 405-410.
Sharma M (2013). Interaction of aflatoxicosis with methionine and zinc levels in diet of broiler chickens. M.V.Sc. Thesis, I.V.R.I., Izatnagar (UP) India. Sharma M, Singh R, Mandal AB and Gupta VP (2014). Efficacy of zinc in amelioration of aflatoxicosis in broiler chickens. Indian Journal of Animal Sciences, 84(3): 311-115.
Shotwell OL, Hesseltine CV, Stubblefield RD and Sorenson WG (1996). Production of aflatoxin on rice. Applied Microbiology, 14: 425-429.
Siegel PB and Gross WB (1980). Production and persistence of antibodies to sheep erythrocytes. 1. Directional selection. Poultry Science, 59: 1-5.
Silambarasan S, Singh R and Mandal AB (2013). Evaluation of the ability of adsorbents to ameliorate the adverse effects of aflatoxin B1 in broiler chickens. Indian Journal of Animal Sciences, 83: 73-77.
Silambarasan S, Singh R and Mandal AB (2015). Efficacy of certain adsorbents on carcass traits and liveability of broiler chickens fed aflatoxin B1 contaminated diet. Indian Journal of Poultry Science, 50(1): 113-117.
Silambarasan S, Singh R and Mandal AB (2016). Evaluation of adsorbents to ameliorate the adverse effects of aflatoxin B1 on blood biochemicals, immune response and histopathology of liver in broiler chickens. Indian Journal of Poultry Science, 50(3): 267-271.
Singh R and Mandal AB (2013). Efficacy of ascorbic acid and butylated hydroxyanisole in amelioration of aflatoxicosis in broiler chickens. Iranian Journal of Applied Animal Science, 3(3): 595-603.
Singh R, Mandal AB and Biswas A (2013a). Efficacy of DLMethionine in amelioration of aflatoxicosis in coloured broiler chicken. Indian Journal of Animal Sciences, 83(12): 1329-1334.
Singh R, Mandal AB and Divya (2015). Efficacy of methionine hydroxy analogue in ameliorating aflatoxicosis in Japanese quails. Animal Nutrition and Feed Technology, 15: 227-234.
Singh R, Mandal AB and Shrivastav AK (2013b). Amelioration of aflatoxicosis in coloured broiler chickens by dietary butylatedhydroxytoluene. Animal Nutrition and Feed Technology, 13: 235-242.
Singh R, Sharma M, Mandal AB and Tyagi PK (2016). Comparative efficacy of DL Methionine vis a vis methionine hydroxy analogue in ameliorating aflatoxicosis in Japanese quails. Indian Journal of Poultry Science, 51(2): 168-173.
Singh R, Shrivastava HP and Shrivastav AK (2010). Mycotoxin contamination in maize as poultry feed. Indian Journal of Poultry Science, 45: 108-110.
Talapatra SK, Ray SC and Sen KC (1940). Estimation of phosphorus, choline, calcium, magnesium, sodium and potassium in feeding stuffs. Journal of Veterinary Science and Animal Husbandry, 10: 243-245.
Thaxton JP, Tung HT and Hamilton PB (1974). Immunosuppression in chickens by aflatoxin. Poultry Science, 53: 721-725.
Tung HT, Wyatt RD, Thaxton P and Hamilton PB (1975). Concentrations of serum proteins during aflatoxicosis. Toxicology and Applied Pharmacology, 34: 320-326.
Virdi JS, Tiwari RP, Saxena M, Khanna V, Singh G, Saini SS and Vadehra DV (1989). Effects of aflatoxin on immune system of the chicken. Journal of Applied Toxicology, 9: 271-275.
Yunus AW, Razzazi-Fazeli E and Bohm J (2011). Aflatoxin B1 in affecting broiler’s performance, immunity, and gastrointestinal tract: A review of history and contemporary issues. Toxins, 3: 566-590.






.jpg&w=3840&q=75)