1 Introduction
As a result of its nutritional properties, wheat (Triticum aestivum L.) is one of the most frequently eaten cereals all over the world by both humans and animals (Vieira, 2006). Brazil is not self-sufficient in the production of this cereal; producing less than half of its consumption needs, and such production is subject to market fluctuations. Because of milder temperatures, the southern region of Brazil accounts for 94.6% of the national production of wheat. However, given the characteristics of the cropping system, the average grain yield in this region is not the highest in Brazil (Empresa Brasileira de Pesquisa Agropecuária, 2013). In addition, the quality of products after wheat processing is directly related to the quality of grains to be processed; therefore, careful cultivation, harvesting and storage of this cereal are crucial (Vieira, 2006).
In plantations, wheat can be contaminated by various diseases because of weather conditions, soil type and crop susceptibility. One of the best-known diseases that commonly affect this cereal is Fusarium head blight, triggered by infection of Fusarium fungi, which not only cause diseases in plants but also produce toxic substances known as mycotoxins through their secondary metabolism (Calori-Domingues et al., 2007).
Mycotoxins are naturally-occurring toxic compounds, produced by a variety of fungal species that grow on agricultural products, during their growth in the field and in storage, as well as in processed food and animal feed (Scussel, 2002). They cause significant economic impact because they reduce plant and animal productivity, and also toxicological impact, with clinical manifestations in both humans and animals (Santos, 2009).
Deoxynivalenol (DON) is a trichothecene mycotoxin, produced mainly by Fusarium graminearum, and it is one of the most frequently found in small cereal grains (Bando et al., 2007; Freire et al., 2007). Production of DON in wheat is considered a virulence factor of F. graminearum (Maier et al., 2006). DON is also known as vomitoxin, because it causes vomiting, especially when consumed by pigs. It is toxicologically relevant, and its synthesis is stimulated by high humidity (Miller, 1995; Beyer et al., 2005), with high incidence in winter cereals, such as wheat and wheat products consumed by humans (Miller, 1995; Mallmann et al., 2003; Calori-Domingues et al., 2007). The World Health Organization (WHO) considers DON as a neurotoxin with a teratogenic nature and immunosuppressive characteristics, and, as trichothecenes in general, it has been associated with chronic and fatal intoxication of humans and animals through consumption of contaminated food (Rotter et al., 1996).
Because the occurrence of fungal infection serves as a warning against mycotoxin contamination, it is worrying that wheat crops harvested in 2014/2015 in southern Brazil were affected by high levels of Fusarium head blight. In this context, the aim of this study was to evaluate the mycotoxicological quality of Brazilian wheat grains and wheat products (wheat flour and wheat bran) for DON contamination during the year of 2014.
2 Materials and methods
DON analyses were conducted on the premises of the Laboratory of Mycotoxicological Analyses (LAMIC), Federal University of Santa Maria - RS, Brazil.
2.1 Samples
A total of 1504 samples of wheat and wheat products (668 of wheat grain, 697 of wheat flour and 139 of wheat bran) were collected from southern Brazil between January and December 2014, and analyzed according they were acquired. The samples were being sold and/or used by cooperatives and agriculture companies.
2.2 Standards and solvents
The DON standard was purchased from Sigma (Sigma-Aldrich, Alcobendas, Spain). Acetonitrile and methanol were purchased from J. T. Baker (Deventer, The Netherlands). All solvents were LC grade. Water was purified with a Milli-Q purification system.
2.3 Preparation of the sample
The toxin was extracted according to the official method no. 986.17 from Association of Official Analytical Chemistry (1995), with some modifications. Three grams of the previously ground sample were extracted in vortex mixers with 24 ml of the solvent mixture containing methanol: water (70: 30, v/v). Sample cleanup was not performed in this method. The extracts of the samples were diluted in a combination of aqueous phase, water: ammonium acetate (995: 5, v/v) and organic phase (water: ammonium acetate: methanol (95: 990: 5, v/v/v), and 5 μL were injected into a liquid chromatography system.
2.4 Determination of DON
DON was determined by separation with high-performance liquid chromatography, and detected by sequential mass spectrometry (LC-MS/MS). The isotopes 13C20-Don and 13C20-Zea were used as internal standard. For quantification of mycotoxins, a seven-point calibration curve was prepared (200, 400, 600, 1000, 2000, 4000 and 8000 µg.Kg-1). A validated method was used for sample preparation, extraction and dilution (LOD: 50 µg.Kg-1; LOQ: 200 µg.Kg-1). For the collected data, it was assumed that the calibration curve showed a linear behavior (r2 >0.9).
2.5 Statistical analysis
The average contamination of all samples and average contamination of positive samples, prevalence and percentage of samples above LMT of DON in wheat grain, wheat flour and wheat bran were calculated in each month of 2014. Tukey test at 5% significance was used to compare the average results of each month. The variables were calculated using Microsoft Excel software (Microsoft Excel 2016 MSO, Microsoft Corporation, Redmond, WA) and analyzed in the statistical program Statgraphics Centurion XV (Statgraphics Centurion 15.2.11, Manugistics Inc., Rockville, MD).
3 Results and discussion
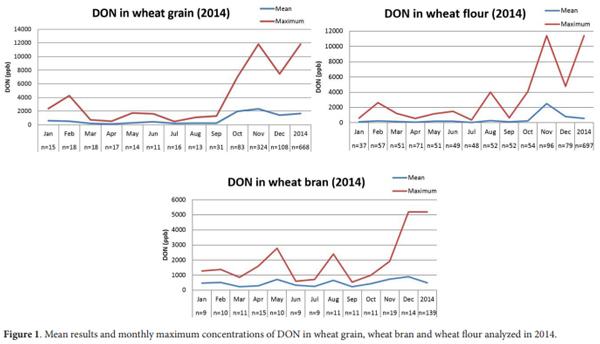
Table 1 shows the results of the occurrence of DON in wheat and wheat products (flour and bran) in 2014. A total of 1000 samples (66.5%) were contaminated with DON, with mean levels of 1574.75 µg.kg-1. Contamination was most common in November with 399 (90.9%) of positive samples and less frequent in April, with 38 (36.9%) positive samples.
A study by Calori-Domingues et al. (2007) evaluated the occurrence of DON in wheat grown in Brazil and imported from abroad. It was found that 94% of the Brazilian wheat and 88% of the imported wheat were contaminated with mean levels of 332 and 90 µg.Kg-1, respectively. In addition, 4% of the Brazilian wheat samples analyzed had contamination levels above 1250 µg.Kg-1, which is the maximum permissible level (MPL) in accordance with European Community law; however, this level is still allowed in Brazil. Although the extent of contamination was greater in that study, the mean levels presented by the authors were below those observed in the period evaluated in the present work, which included samples from crops containing high levels of Fusarium head blight (in the second semester).
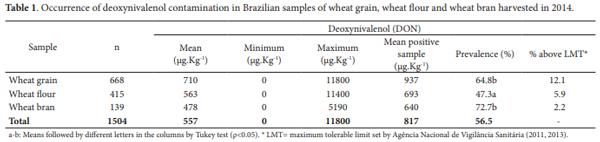
Wheat flour was the fraction showing the lower prevalence, while wheat grain and bran presented the highest prevalence of DON. A study by Nowicki et al. (1988) showed that the distribution of DON in ground wheat grain fractions is influenced by the degree of fungal penetration into the grain endosperm, and this susceptibility is dependent on plant variety. Those authors found that when penetration was low, higher levels of infection and DON were found in the grain surface and, consequently, low concentrations of DON were recovered in the flour.
A more recent study indicated that in the post-harvest stage, the cleaning, aeration, debranning and milling procedures have an influence in the distribution of mycotoxins in wheat fractions. In the milling process, there is no mycotoxin reduction, although mycotoxin concentrations may be redistributed and concentrate according to the milling fractions. Mycotoxins tend to be concentrated in outer fractions intended for animal feed (bran, flour shorts screenings and middlings) and lower in inner fractions intended for human consumption (flour or semolina) (Cheli et al., 2013).
On the other hand, the not significant difference between the fractions contamination is not in accordance with findings reported in the literature (Nowicki et al., 1988; Samar et al., 2003; Lancova et al., 2008). However, it should be noted that the samples analyzed in this study were independent, and there is no interrelationship between the whole grain and the different fractions. In addition, knowing this survey deals with samples from two different seasons, involving different wheat cultivars yielded in 2014 and naturally showing different sensitivity to FHB, the variation in levels of contamination may have influenced the average results of DON and consequently the statistical results. Twenty one percent (21% n = 145) of the analyzed wheat grain samples had contamination levels above 2000 µg.kg-1 (the maximum permissible level for wheat grain for further processing by current Brazilian law (Agência Nacional de Vigilância Sanitária, 2011, 2013), and the highest level of contamination found for the samples was 11800 µg.kg-1, confirming the low mycotoxicological quality of this cereal from the year 2014. Among the wheat flour samples, 10% (n = 71) were out of the limits currently allowed by Brazilian regulation.
According to the Agriculture Federation of Rio Grande do Sul (FARSUL), as a result of the legislation for mycotoxins (enforced in Brazil since 2011), if the allowed limit (1750-2000 µg.Kg-1) is exceeded, wheat is not suitable for either human or animal consumption. It should be noted that new values will take effect as of 2017. The same organization also estimates that one million out of the 1.7 million tons produced in the last harvest in Rio Grande do Sul would be affected, and 700,000 tonnes of low quality wheat had been traded until December 9th, 2014 at low prices to international markets willing to absorb this production with low mycotoxicological quality (Batchold, 2015).
Figure 1 shows the mean of monthly concentration and the maximum level found in the evaluated samples of wheat and wheat products. The highest mean of DON contamination was in November when the samples of the new wheat crop started to be introduced (2014/2015).
According to Companhia Nacional de Abastecimento (2015), in the 2014/2015 harvest in Rio Grande do Sul, the area sown with wheat was as big as 1.14 million hectares, which represents an increase of 9.8% over the previous harvest. However, the same government agency informed that during the growth of the crop, several adverse factors - frost, torrential rains, floods, lack of light, excessive heat, disease attacks in general, hail and lodging - influenced the end result.
Buerstmayr et al. (2009) reported that in Southern Brazil (latitude 28 S and 23 S), where wheat crops are located, the main limiting factor for wheat production is the high relative humidity, which favors the occurrence of outbreaks of fungal diseases, especially Fusarium Head Blight (FHB). This disease is influenced by warm temperatures and rainy days during the flowering time, causing losses in grain yield and reductions in baking and seed quality.
Within this framework, Rio Grande do Sul produced 1.5162 thousand tons in 2014, 52.3% less than the harvest in 2013, reflecting a decrease by 56.5% in productivity (Companhia Nacional de Abastecimento, 2015).
Several studies worldwide (Love & Seitz, 1987; Miller, 1995; Trigo-Stockli et al., 1995; Moschini & Fortugno, 1996; Osborne & Stein, 2007) have correlated periods of high humidity with high incidence of Fusarium head blight in wheat crops, which consequently tends to cause higher levels of DON in wheat and wheat products (Lori et al., 2003; Vanheule et al., 2014), as noted in our study in the final quarter of 2014.
4 Conclusions
Considering wheat as a raw material widely used in the food industry and the high levels of DON contamination observed in certain months of the year, especially in samples from the harvest presenting high incidence of Fusarium head blight, it is crucial to carry out appropriate mycotoxicological monitoring of the quality of wheat and wheat products traded to prevent the population exposition to levels above those allowed by regulation.
This article was originally published in Food Science and Technology, Campinas, 37(1): 8-12, Jan.-Mar. 2017. http://dx.doi.org/10.1590/1678-457X.05915. This is an Open Access article distributed under the terms of the Creative Commons Attribution License.
References
Agência Nacional de Vigilância Sanitária – ANVISA. (2011, March 9). Dispõe sobre os limites máximos tolerados (LMT) para micotoxinas em alimentos (Resolução RDC nº 7, de 18 de fevereiro de 2011). Diário Oficial [da] República Federativa do Brasil.
Agência Nacional de Vigilância Sanitária – ANVISA. (2013, December 30). Dispõe sobre a prorrogação dos prazos estabelecidos para os limites máximos tolerados para micotoxinas em alimentos (Resolução RDC nº 59, de 26 de dezembro de 2013). Diário Oficial [da] República Federativa do Brasil.
Association of Official Analytical Chemistry – AOAC. (1995). Official methods (16th ed., chap. 49, pp. 1-49). Washington: AOAC.
Bando, E., Gonçales, L., Tamura, N. K., & Machinski, M. (2007). Biomarcadores para avaliação da exposição humana às micotoxinas. Jornal Brasileiro de Patologia e Medicina Laboratorial, 43(3), 175-180. http://dx.doi.org/10.1590/S1676-24442007000300006.
Batchold, F. (2015, December 23). Gaúchos exportam trigo contaminado. Folha de São Paulo. Retrieved from http://www1.folha.uol.com.br/ mercado/2014/12/1566263-gauchos-exportam-trigo-contaminado. shtml
Beyer, M., Verreet, J. A., & Ragab, W. S. M. (2005). Effect of relative humidity on germination of ascospores and macroconidia of Gibberella zeae and deoxynivalenol production. International Journal of Food Microbiology, 98(3), 233-240. PMid:15698684. http://dx.doi. org/10.1016/j.ijfoodmicro.2004.07.005.
Buerstmayr, H., Ban, T., & Anderson, J. A. (2009). QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: a review. Plant Breeding, 128(1), 1-26. http://dx.doi. org/10.1111/j.1439-0523.2008.01550.x.
Calori-Domingues, M. A., Almeida, R. R., Tomiwaka, M. M., Gallo, C. R., Gloria, E. M., & Dias, C. T. S. (2007). Ocorrência de desoxinivalenol em trigo nacional e importado utilizado no Brasil. Food Science and Technology, 27(1), 181-185. http://dx.doi.org/10.1590/S0101- 20612007000100032.
Cheli, F., Pinotti, L., Rossi, L., & Dell’Orto, V. (2013). Effect of milling procedures on mycotoxin distribution in wheat fractions: a review. LWT - Food Science and Technology, 54(2), 307-314. http://dx.doi. org/10.1016/j.lwt.2013.05.040.
Companhia Nacional de Abastecimento – CONAB. Acompanhamento da safra brasileira: grãos (Vol. 2 - Safra 2014/15, No. 4 - Quarto Levantamento). Brasília: CONAB; 2015.
Empresa Brasileira de Pesquisa Agropecuária – EMBRAPA. (2013). Annual wheat newsletter (Vol. 59 III). Brasília: EMBRAPA.
Freire, F., Vieira, I., Guedes, M., & Mendes, F. (2007). Micotoxinas: importância na alimentação e na saúde humana e animal (Documentos, No. 110, 48 p.). Fortaleza: Embrapa Agroindútria Tropical.
Lancova, K., Hajslova, J., Kostelanska, M., Kohoutkova, J., Nedelnik, J., Moravcova, H., & Vanova, M. (2008). Fate of trichothecene mycotoxins during the processing: milling and baking. Food Additives & Contaminants: Part A, 25(5), 650-659. PMid:18473219. http:// dx.doi.org/10.1080/02652030701660536.
Lori, G. A., Sisterna, M. N., Haidukowski, M., & Rizzo, I. (2003). Fusarium graminearum and deoxynivalenol contamination in the durum wheat area in Argentina. Microbiological Research, 158(1), 29-35. PMid:12608577. http://dx.doi.org/10.1078/0944-5013-00173.
Love, G. R., & Seitz, L. M. (1987). Effects of locations and cultivar on Fusarium head bligth (scab) in wheat from Kansas in 1982 and 1983. Cereal Chemistry, 64, 124-128.
Maier, F. J., Miedaner, T., Hadeler, B., Felk, A., Salomon, S., Lemmens, M., Kassner, H., & Schafer, W. (2006). Involvement of trichothecenes in fusarioses of wheat, barley and maize evaluated by gene disruption of the trichodiene synthase (Tri5) gene in three field isolates of different chemotype and virulence. Molecular Plant Pathology, 7(6), 449-461. PMid:20507460. http://dx.doi.org/10.1111/j.1364- 3703.2006.00351.x.
Mallmann, C. A. M., Mürman, L., Dilkin, P., & Almeida, C. A. A. (2003). Avaliação da contaminação por desoxinivalenol em trigo utilizado na alimentação humana. In Anais do 1 Congresso Brasileiro de Farmácia, São Paulo, Brasil.
Miller, J. D. (1995). Fungi and mycotoxins in grains: implications for stored product research. Journal of Stored Products Research, 31(1), 1-16. http://dx.doi.org/10.1016/0022-474X(94)00039-V.
Moschini, R., & Fortugno, C. (1996). Predicting wheat head blight incidence using models based on meteorological factors in Pergamino, Argentina. European Journal of Plant Pathology, 102(3), 211-218. http://dx.doi.org/10.1007/BF01877959.
Nowicki, T. W., Gaba, D. G., Dexter, J. E., Matsuo, R. R., & Clear, R. M. (1988). Retention of the Fusarium mycotoxin deoxynivalenol in wheat during processing and cooking of spaghetti and noodles. Journal of Cereal Science, 8(2), 189-202. http://dx.doi.org/10.1016/ S0733-5210(88)80029-8.
Osborne, L. E., & Stein, J. M. (2007). Epidemiology of fusarium head blight on small-grain cereals. International Journal of Food Microbiology, 119(1-2), 103-108. PMid:17716761. http://dx.doi. org/10.1016/j.ijfoodmicro.2007.07.032.
Rotter, B. A., Prelusky, D. B., & Pestka, J. J. (1996). Toxicology of deoxynivalenol. Journal of Toxicology and Environmental Health, 48(1), 1-34. PMid:8637056. http://dx.doi.org/10.1080/009841096161447.
Samar, M. M., Fontán, C. F., Resnik, S. L., Pacin, A. M., & Castillo, M. D. (2003). Distribution of deoxynivalenol in wheat, wheat flour, bran, and gluten, and variability associated with the test procedure. Journal of AOAC International, 86(3), 551-556. PMid:12852575.
Santos, J. S. (2009). Aplicação biotecnológica: anticorpo monoclonal anti-desoxinivalenol para monitoramento e avaliação da exposição pelo consumo de trigo (Triticum aestivum L.) (Master’s thesis). Universidade Estadual de Londrina, Londrina.
Scussel, V. M. (2002). Fungos e micotoxinas associados a grãos armazenados. In I. Lorini, L. H. Miike & V. M. Scussel (Eds.), Armazenagem de grãos (pp. 674-804). Campinas: Instituto Bio Geneziz. Seção 9.
Trigo-Stockli, D. M., Curran, S. P., & Pedersen, J. R. (1995). Distribution and occurrence of mycotoxins in 1993 Kansas Wheat. Cereal Chemistry, 72, 470-474.
Vanheule, A., Audenaert, K., De Boevre, M., Landschoot, S., Bekaert, B., Munaut, F., Eeckhout, M., Höfte, M., De Saeger, S., & Haesaert, G. (2014). The compositional mosaic of Fusarium species and their mycotoxins in unprocessed cereals, food and feed products in Belgium. International Journal of Food Microbiology, 181, 28-36. PMid:24806576. http://dx.doi.org/10.1016/j.ijfoodmicro.2014.04.012.
Vieira, S. M. (2006). Química dos cereais. Fortaleza: SENAI-CE/CERTREM.











.jpg&w=3840&q=75)