Continuing livestock production from endophyte-infected tall fescue and perennial ryegrass: recent studies with a toxin adsorbent
To date, dopamine antagonists and toxin adsorbing materials offer the most potential for alleviating toxicity and simultaneously increasing animal performance. Further research will determine if this potential becomes reality.
Introduction
Tall fescue is the most abundant and economically important cool season grass grown in the United States. Perennial ryegrass, the most important grass species grown in New Zealand, is also important to the livestock industry of Australia, the United Kingdom, and South America (Rowan, 1993).
Although considered a high-quality forage from a nutrient composition standpoint, animal performance in tall fescue grazing systems is frequently suboptimal because of the endophytic fungus, Acremonium coenophialum, which inhabits the intercellular spaces of the tall fescue leaf sheaths.
Hoveland (1993) estimated that the US beef industry loses $600 million annually because of slow weight gains and reproductive inefficiency of cattle grazing fescue. Ryegrass staggers, a common neurological disorder of sheep grazing perennial ryegrass, has been linked to infection of the grass plant by the endophytic fungus Acremonium lolii.
Prestidge et al. (1991) estimated ryegrass staggers costs the New Zealand livestock industry over $40 million annually. This review will address (1) the mutualistic symbiosis of grass and fungus; (2) the components of the fungal endophytes that cause fescue toxicity and ryegrass staggers; (3) the effects of the resulting metabolic disorders; and (4) efforts to counteract these disorders.
Symbiosis
No external fructification or spores are produced by either A. coenophialum or A. lolii as they complete their life cycle in their grass hosts (Bacon et al., 1986). Instead, the endophytes rely on high concentrations of sucrose and other sugars in the meristematic regions of the leaf sheath in the host grass for all their required nutrients (Joost, 1995).
The slow growing fungal mycelia proliferate into the developing seedhead as the terminal stem meristem elevates to produce the grass inflorescence. Mycelia then multiply in the nutrientrich nucellus region of the ovule and ultimately infect the developing seed.
When the seed germinates, mycelia spread into the intercellular spaces of the leaves of the new grass plant. In this way, the endophyte relies entirely on its grass host for dissemination and proliferation. Thus, the grass host provides the fungal symbiont with nutrition and reproductive viability, while the endophytic fungus protects its grass host from abiotic and biotic stresses.
The discovery that the Acremonium genus was related to poor performance and staggers of animals consuming endophyte-infected (E+) tall fescue and perennial ryegrass led to a rapid development of endophyte-free (E-) grass cultivars. However, subsequent investigations showed E- tall fescue stands were less persistent than E+, especially when subjected to drought conditions (Read and Camp, 1986). Almost as soon as E- perennial ryegrass stands were established, they were killed by the Argentine stem weevil (Prestidge et al., 1982) because they lacked the resistance to this pest imparted on E+ ryegrass by A. lolii metabolites.
Pinkerton et al. (1990) found E+ grasses had higher germination rates because excessive water uptake by E- seed during imbibition reduced final germination. The higher germination rate of E+ seeds allows denser seedling distribution in tall fescue and perennial ryegrass stands. The presence of endophyte also enhances tillering (DeBattista et al., 1990) during the first 10 weeks of growth, which, in turn, increases ground cover (Clay, 1987) and decreases the potential for weed invasion (Prestidge et al., 1992). The competitive advantage for E+ plants may result from a gradual shift from low endophyte infestation frequency to nearly complete dominance by E+ plants as E- plants succumb to environmental stresses (Clay, 1993).
The competitiveness of E+ plants can be detrimental in clover establishment. Sutherland and Hoglund (1990) found E+ perennial ryegrass reduced the survival of white clover relative to Estands and Prestidge et al. (1992) concluded that the reduced survival was due to competition for light, water, and nutrientsnot an alleopathic effect of ryegrass exudates. Other research has demonstrated the greater susceptibility of E- fescue to biotic stresses that reduce survivability of pure stands. Hardy et al. (1986) reported fall army worms preferred E- tall fescue leaf tissue, Pedersen et al. (1988) found E+ tall fescue was resistant to root feeding nematodes, and Welty et al. (1991) reported E+ tall fescue had a greater resistance to many plant diseases, but crown rust was an exception.
The grazing animal presents a biotic stress that can affect plant persistence. Dry matter intake of cattle grazing E+ tall fescue was 24 to 44% lower than when grazing E- (Stuedemann et al., 1989). Much of this difference was attributed to decreased grazing of E+ tall fescue during daylight hours brought about by the low heat tolerance of the grazing animals (Coffey et al., 1992; Howard et al., 1992). Seman et al. (1990) found animals grazing E+ tall fescue spent more time in the shade during the heat of the day than those grazing E-. These same animals failed to compensate for this reduction with increased nighttime grazing. Simultaneous morphological changes in E+ fescue, presumed to be a result of the endophyte, may protect the fescue plant from overgrazing.
Toxic fungal components
The nonpathogenic, intercellular and entirely seedborne fungal endophytes of Acremonium coenophialum and A. lolii (Bacon, 1994) produce the mycotoxins responsible for the symptoms of fescue toxicosis and ryegrass staggers. The mycotoxins of primary focus are the ergot alkaloids, which include clavines, ergolene acids, lysergic acids and lysergic acid amide derivatives, ergopeptides and ergopeptine alkaloids (Garner et al., 1993). Table 1 lists the toxins and suspected toxins found in Acremonoid symbiota (Bacon, 1994; Porter, 1994).
Table 1. Toxins and suspected toxins in tall fescue and perennial ryegrassa.
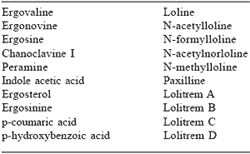
aBacon, 1994; Porter, 1994.
Ergovaline is the primary ergopeptine alkaloid associated with fescue toxicosis (Porter, 1995), but other alkaloids, including the loline alkaloids Nacetylloline and N-formylloline, may contribute to the disorder (Bush et al., 1982, 1993; Yates et al., 1990). Although peramine has been investigated as a potential cause of fescue toxicosis, no evidence of mammalian toxicity has been associated with this alkaloid (Porter, 1994). When Hemken et al. (1979) investigated strains of tall fescue-ryegrass hybrids selected for high and low levels of perloline, they found an increased incidence of fescue toxicosis in dairy cows and lambs consuming the low perloline strain. Bush et al. (1982) found the low perloline strain used by Hemken et al. (1979) contained higher levels of N-acetylloline and N-formylloline than the low perloline strain, leading both groups of researchers to conclude that lolines may have contributed to the observed toxicity. Porter (1994) further concluded that these loline alkaloids were produced by the plant either in response to the fungal infection, as defense against insect herbivory, or both. Porter (1995) later concluded these alkaloids may augment the toxicity of ergot alkaloids without being directly related to animal behavior. Neither N-acetylloline nor N-formylloline have been identified in A. lolii infected ryegrass. Therefore, ergovaline has been identified as the primary marker associated with A. coenophialum infected fescue, although fescue toxicosis observed in livestock is probably caused by the total concentration of ergot alkaloids and possibly other alkaloids in the grass (Porter, 1995).
The alkaloids lolitrem and paxilline are considered the primary toxic agents in perennial ryegrass (Porter, 1994). Lolitrem B, a tremorgenic neurotoxin, appears to be the primary cause of ryegrass staggers (Rowan, 1993) and is the main ergopeptine found in perennial ryegrass (Porter, 1995). Lolitrem levels are negligible during winter, rise during spring and summer, and reach a peak in autumn (Rowan, 1993). They are concentrated in the basal leaf sheaths where concentrations of fungal mycelia are highest. This area of concentration coincides with a high occurrence of ryegrass staggers when animals graze down to the base of pasture plants (Rowan, 1993).
Toxicity has been reported to occur in animals consuming A. coenophialum – infected tall fescue when levels of ergovaline reach 50 ng/g of grass. Perennial ryegrass infected with A. lolii produces the staggers syndrome when lolitrem B concentrations reach 5 μg/g of grass. The diversity of biologically active alkaloids present in E+ grasses makes it difficult to pinpoint specific compounds that cause fescue toxicosis or ryegrass staggers and probably reduces the total quantity of toxin (or alkaloids) necessary to precipitate these syndromes. Therefore total, instead of individual, alkaloid concentrations should be determined before livestock graze E+ pastures.
Fungal effects on animal performance
TALL FESCUE
Even though tall fescue affords numerous agronomic advantages, Pratt and Haynes (1954) showed animal performance failed to reach levels that would be predicted from nutrient analyses. Results from experiments conducted in ten southeastern US states showed that decreased gains in animals consuming E+ tall fescue were uniform and not limited to geographical location or pasture management system (Table 2). Hoveland et al.
(1984) reported that reduced gains of steers consuming E+ tall fescue can occur anytime during the year. Garner et al. (1984) and Crawford et al. (1989) estimated for each 10% increase in endophyte infestation there was a 0.045 kg/hd decrease in steer ADG. Although consumption of ergovaline, the ergopeptide present in greatest concentration in E+ tall fescue, is highest in May, forage loline concentrations peak in July and August. Signs of toxicity can occur in June, July and August, suggesting an interaction between endophyte level and environmental temperature (Hemken et al., 1981).
Table 2. Effect of endophyte level on gains of steers grazing tall fescue a,b
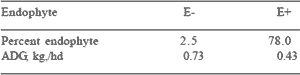
a,bAdapted from Paterson et al. (1995)
bSummary of ten experiments in ten southeastern U.S. states.
Fewer data are available comparing cows grazing E+ with those grazing E- tall fescue. Table 3 summarizes cow/calf data reported between 1983 and 1993. In general, cows grazing E+ lost more weight, had decreased milk production and pregnancy rates, and weaned lighter weight calves than cows grazing E-tall fescue. Other work attributed decreased calf weaning weights to decreased milk production of cows grazing E+ fescue (Ashley et al., 1987; Keltner et al., 1989).
Peters et al. (1992) reported a 25% reduction in milk production for cows grazing E+ when compared with those grazing E- tall fescue. Danielson et al. (1986) estimated that for each 10% increase in pasture endophyte infection there was a 0.15 kg/day decrease in milk production. Also, cows consuming E+ forage typically have lower conception rates than those grazing E- fescue (Beers and Piper, 1987; Gay et al., 1988; Schmidt et al., 1986). These researchers attributed lower conception rates directly to endophyte toxicity and(or) indirectly to increased weight and body condition loss, both of which can result from consumption of E+ fescue.
Table 3. Effect of endophyte infection of tall fescue on cow/ calf performance a
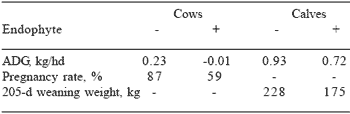
aAdapted from Paterson et al. (1995)
Stocker calves, as well as feeder calves and yearlings, are usually discriminated against at marketing because of rough hair coats, high respiration rates, lack of body condition, and appearance of general ill-thrift. However this discrimination may be unfounded because Forcherio et al. (1992) demonstrated that steers previously grazed on E+ in the spring and summer had higher feedlot daily gain (1.40 kg/hd) than those previously grazed on E- fescue (1.27 kg/hd). Data collected by Cole et al. (1987) and Lusby et al. (1990) showed that stockers and feeders produce enough compensatory gain during the first two months after removal from E+ fescue pastures to conclude that endophyte does not have a permanent effect on post-grazing growth rate.
Much of the reduction in performance of animals consuming E+ fescue has been attributed to reduced forage intake. Fribourg et al. (1991) reported that cattle prefer E- to E+ forage. Peters et al. (1992) showed cows grazing E+ had organic matter intakes similar to those grazing E- tall fescue or orchardgrass during June (2.6% body weight). But, during August, when environmental temperatures were higher, cows on E+ pastures consumed less (1.6% body weight) than those on either E- fescue or orchardgrass (2.0% body weight). Even with similar levels of intake in June, cows consuming E+ lost weight while those grazing E- fescue gained weight. Goetsch et al. (1987) found dry matter intake decreased linearly as animals were offered increasing amounts of E+ forage. However, the decrease was not the result of nutritional factors that typically control dietary forage intake. Forcherio et al. (1992) reported similar organic matter digestibilities when E+ and E- hays were fed to heifers. Sheep fed diets containing higher than normal levels of ergovaline, through E+ seed, digested less than normal amounts of fiber because of higher than normal ruminal fluid dilution and outflow rate (Hannah et al., 1990).
Cattle on E+ pastures graze more at night than those grazing E- fescue (Bond et al., 1984; Paterson et al., 1987), but fail to compensate for reduced daylight grazing. Stuedemann et al. (1985) found steers consuming E- fescue spent 60% of their time grazing from 1200 to 1800 hrs, whereas E+ steers spent only 4 to 6% of that time grazing. Howard et al. (1992) observed steers grazing E+ fescue spent more time standing idly while E- steers spent their time grazing or lying down. Steers preferred clover in E+ mixed pastures, but fescue was preferred over clover in E- mixed pastures (Fribourg et al., 1991).
Hemken et al. (1984) described the following symptoms associated with fescue toxicosis in cattle: reduced growth rate, lower milk production, decreased feed intake, and(or) increased respiration rate. Rough hair coats, elevated rectal temperatures and decreased gains were reported by Read and Camp (1986). Hurley et al. (1981) and Schillo et al. (1988) showed decreased serum prolactin level and Schmidt and Osborn (1993) observed excessive salivation in association with the consumption of E+ tall fescue. Hemken et al. (1981) found calves consuming E+ tall fescue consumed less dry matter, had higher rectal temperatures and respiration rates, and gained slower than calves grazing less toxic tall fescue, especially when ambient temperatures exceeded 31oC.
PERENNIAL RYEGRASS
Grazing sheep, cattle, horses and deer are affected by A. lolii infected ryegrass (Prestidge, 1993). Symptoms of this infection include increased rectal temperatures and respiration rates, depressed serum prolactin levels and increased incidence of scours (Bluett et al., 2001). Animals with ryegrass staggers exhibit tetanic muscle spasms, which result in severe incoordination and hypersensitivity to external stimuli (Rowan, 1993). Muscle spasms usually begin with head tremors and a slight trembling in the neck and limbs. Incoordination, head bobbing and swaying may occur while standing. If disturbed in this state, the animal moves with jerky movements. Severely afflicted animals may prance on their fore, hind, or all four limbs, followed by collapse and severe muscle spasms lasting 5 to 10 seconds. Animals then rise and walk away as if unaffected (Prestidge, 1993).
Counteracting endophytic disorders
Research efforts in the 1970s and 1980s yielded a mass of data demonstrating the dramatic negative effects of E+ tall fescue consumption on animal performance. It is not surprising these effects were found to be temperature related (Seman et al., 1990), especially when one considers that tall fescue, a cool season grass, is grazed during the hot summer. Bush and Buckner (1973) concluded the decreased animal performance accompanying consumption of E+ fescue was greater than chemical analyses of the forage/grass predicted. Since then, major efforts have been expended towards alleviating some, or all, of the toxic effects associated with decreased performance of animals consuming E+ tall fescue. Efforts have been directed toward both the plant and the animal in attempts to align performance with nutrient composition.
TREATMENT OF FESCUE WITH MEFLUIDIDE, A PLANT GROWTH REGULATOR
The data in Table 4 (Garrett et al., 1988) substantiate the earlier conclusion of Bush and Buckner (1973) that animal performance may not be accurately predicted from fescue forage composition, especially if the endophyte is allowed to be assertive. Yearling steer gain from May 7 to September 24 was 188 kg/ha. Sixty percent of the total gain was attained from May 7 to June 4, when crude protein content of the forage was decreasing and fiber components were increasing. In contrast, only 2% of the total gain was attained from August 27 to September 24, the period when forage crude protein was highest and fiber components were lowest. Garrett et al. (1988) compared these data with those obtained from E+ fescue that had been sprayed with mefluidide, a chemical plant growth regulator, on April 15. Forage crude protein levels (Table 5) remained 50% higher in the treated fescue than in nontreated (Table 4). Concurrently, average NDF and ADF concentrations were only 91 and 80%, respectively, of the concentrations in nontreated fescue. Total steer gains were 26% higher with mefluidide-treated fescue and tended to decrease as forage CP decreased. Ely et al. (1985) found changes in cow/calf performance (calf gain, maintenance of cow weight, and milk production) could be invoked by altering the nutrient composition of E+ fescue through plant growth regulation. These results indicate animal performance can be related to the nutrient composition of the forage if some, or all, of the endophytic effects are alleviated.
Table 4. Chemical composition and steer gains from KY 31 tall fescue a,b.
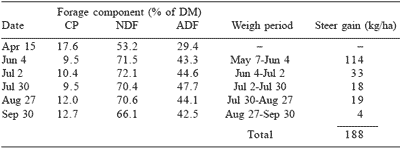
aGarrett et al. (1988)
b90% endophyte-infected
Table 5. Effects of treating endophyte-infected tall fescue pastures with mefluididea.
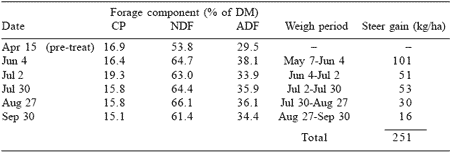
aGarrett et al. (1988)
Many non-performance indicators have been developed to measure how animals respond to E+ tall fescue consumption. Some of these are rectal temperature, tympanic temperature, respiration and heart rates, blood cholesterol, free fatty acids, copper, cortisol, prolactin, epinephrine and norephinephrine concentrations. Likewise, numerous experiments have demonstrated positive or negative responses, as measured by non-performance indicators, to a wide variety of animal supplements or treatments. These include energy, protein, mineral, and(or) vitamin supplementation, molasses-based complete supplements, Aspergillus oryzae fermentation extract, antibiotics, and dopamine antagonists (Paterson et al., 1995). Any one of these materials may elicit a positive response within a small experimental window of fescue toxicity. However, when applied in practical grazing situations, often overall animal performance response (gain and/or milk production) is nonsignificant even though the non-performance indicator response is significant.
A DIETARY TOXIN ADSORBENT
Yeasts have been used as high-quality protein in animal diets for many years (Evans and Dawson, 2000). The vitamin, enzyme and cofactor content also make them useful digestive aids in ruminant and nonruminant diets (Dawson, 1994). Girard (1996) showed that positive effects of live yeast cultures on animal production were associated with specific yeast metabolites. More recent evidence suggests that other positive effects, such as mycotoxin binding, may be associated with certain fractions of the yeast cell wall (Evans and Dawson, 2000). Alltech Inc. has developed a product from the cell wall fraction of Saccharomyces cerevisiae (Evans and Dawson, 2000) that adsorbs a range of fungal mycotoxins (Devegowda et al., 1996).
A second generation product of the yeast cell wall preparation was tested in vitro for its ability to bind ergotamine, which has a structure similar to ergovaline (Chestnut et al., 1992). A series of in vitro experiments further demonstrated ability of the yeast cell wall preparation to bind significant amounts of one of the toxins associated with fescue toxicosis in simulated gastrointestinal environments developed by Evans and Dawson (2000). Based on these demonstrations, the University of Kentucky is currently conducting a study in which beef cow/ calf pairs graze KY 31 tall fescue from early May to late October. The objective of the 2-yr study is to determine the potential for this product to adsorb toxins contained in the E+ forage. In the first year (2001), 92 Angus and Angus x Beefmaster cow/ calf pairs were allotted to nine, 10.5-ha fescue pastures (>90% infected). Three replicate pastures were randomly assigned to one of three treatments. The negative control group received no supplement, the positive control group was given 0.45 kg/hd/ day ground shelled corn, and the test group received 0.45 kg/hd/day of a supplement comprised of 95.6% ground shelled corn and 4.4% of the test compound (modified yeast cell wall preparation, MYCP).
Lactating cow performance during the 2001 grazing season is summarized in Table 6. Cows in the negative control and MYCP treatments gained more weight (P<0.10) than positive control cows from May 2 to July 12. Simultaneously, cows on the positive control treatment lost body condition while those on the negative control remained the same and cows given the test substance gained condition. The forage crude protein during June (8.1%) was lower than any other time during the grazing season. Garrett and co-workers reported similar crude protein concentrations of forage samples collected from adjacent pastures in 1988. Forage ergovaline concentrations were also highest in June, 2001.
Cows consuming the MYCP test substance gained more than those in the negative and positive control treatments from July 12 to October 22 and for the total 175-day grazing season (P<0.10). Most of the overall gain advantage (May 2 to October 22) was attained during the July 12 to October 22 period. Paterson et al. (1995) reported that ergovaline concentrations were highest in May, but toxicity symptoms were more pronounced in July and August. The increased gain of MYCP cows during this period tends to support the theory of Evans and Dawson (2000) that MYCP may adsorb some endophytic toxins of fescue and allow cows to gain more weight and body condition. Body condition scores shown in Table 6 substantiate the gain data in that MYCP cows gained more body condition than negative or positive control cows from July 12 to October 22 (P<0.05). Cows consuming the MYCP treatment gained 0.4 condition score from May 2 to October 22, whereas negative and positive control cows gained only 0.1 during the 175-day study.
Table 6. Performance of lactating cows grazing endophyteinfected KY 31 tall fescue and supplemented with MYCP (University of Kentucky, 2001).
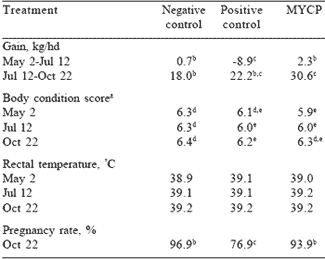
a1=emaciated, 10=obese
b,cMeans in row differ (P<0.10)
d,eMeans in row differ (P<0.05)
No treatment differences were found for rectal temperatures on any date. Pregnancy rates were similar for negative control and MYCP cows, but lower for those in the positive control treatment. Three of the four open cows in the positive control treatment were in one pasture. Although all bulls passed a breeding soundness exam immediately prior to the start of the breeding season (May 4), finding 30% of the cows in a single positive control pasture to be open may be attributed to an unknown low fertility of the bull. Calf daily gain was not different any time during the 175-day grazing season (0.89, 0.85, and 0.91 kg/hd for negative control, positive control and MYCP, respectively).
Tympanic temperatures were continuously monitored in 27 cows (three per pasture) during three, 3-day periods (June 14-17, July 17-20, and August 20-23) of the Kentucky 2001 grazing study. Temperatures were recorded on a data logger attached to a therimistor cable inserted into the ear canal within 2 cm of the tympanic membrane (Paul, 1999). These data are presented in Table 7.
Maximum and minimum ambient temperatures were 30.6, 19.2; 30.5, 19.7; and 28.1 16.1 oC for the June, July, and August periods, respectively. Cows in the MYCP treatment tended to have lower maximum temperatures than negative controls in each period, although no treatment differences were found for minimum temperatures. Diurnal ranges (maximum–minimum) and partial differences (maximum–average) were less (P<0.05) for MYCP than negative control cows in June and August. Although not statistically different, the diurnal range and partial difference in July were numerically lowest for MYCP cows. Paul (1999) used the same tympanic temperature monitoring system to conclude artificial shades were effective in lowering core body temperature. The data in Table 7 indicate that MYCP may adsorb endophytic toxins and thereby keep maximum core body temperatures from soaring to toxic levels when ambient temperatures are high. If dissipation of excess body heat is not required, productivity can be improved.
Table 7. Tympanic temperatures (°C) of cows grazing endophyte-infected KY 31 tall fescue and supplemented with MYCP (University of Kentucky, 2001)
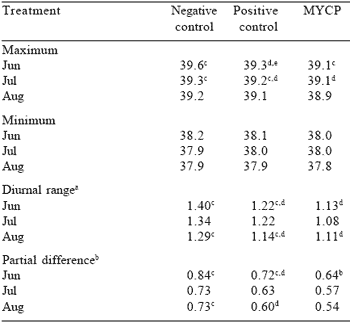
aMaximum minus minimum temperature
bMaximum minus average temperature
c,d,eMeans in a row differ (P<0.05)
Another 2001 University of Kentucky study hypothesized that MYCP supplementation would increase the fecal toxin excretion when E+ tall fescue seed was consumed. To test this hypothesis, twelve Holstein steers, weighing 160 to 270 kg, were gradually acclimatized to a temperature-controlled room for 7 days by gradually raising the room temperature to 30 °C. Steers were maintained in individual tie stalls under 16 hr of daylight and 8 hr of darkness and had continual access to fresh water.
An adjustment diet of cracked corn, cottonseed hulls, crimped oats, soybean meal and a mineral/vitamin mix was fed during the adjustment period. Following this period steers were weighed, blocked by weight and assigned to four treatments: (1) E+ KY 31 tall fescue seed, (2) E+ KY 31 tall fescue seed plus MYCP, (3) E- Jesup tall fescue seed, and (4) EJesup tall fescue seed plus MYCP. All calves received the adjustment diet and fescue seed. The level of MYCP added to treatments (2) and (4) was the same as used in the 2001 Kentucky grazing study (44 g/kg). Steers were fed diets once daily at 0800 at a rate to ensure 5% orts. Fecal grab samples (~100 g) were collected at 0830 every third day of the 21-day trial and analyzed for ergovaline and ergovalinine. Concentrations of dietary ergovaline in treatments (1), (2), (3) and (4) were 0.92, 0.95, 0.13, and 0.17 μg, respectively. Because diets (3) and (4) had low concentrations of ergovaline, fecal samples from only treatments (1) and (2) were analyzed for ergovaline and ergovalinine.
Fecal concentrations of total ergot alkaloids (ergovaline and ergovalinine) are shown in Figure 1. The total alkaloid excretion was higher for diet (1) than (2) during the first 18 days of the trial. Because these diets contained high levels of toxins, it is theorized that MYCP consumed in treatment (2) became saturated after 6 days and could not adsorb any additional toxins thereafter. These data show MYCP does adsorb ergovaline and ergovalinine in the gastrointestinal tract of steers and increases fecal alkaloid excretion. Results also tend to indirectly agree with those of the 2001 grazing study and directly agree with the in vitro work of Evans and Dawson (2000), in which a similar cell wall derivative was able to bind ergotamine, a compound that is structurally similar to ergovaline (Chestnut et al., 1992).
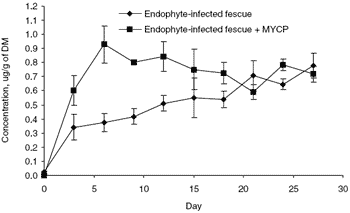
Figure 1. Means of fecal total ergot alkaloid (ergovaline and ergovalinine) concentration of steers fed endophyte-infected tall fescue seed with or without modified yeast cell wall preparation (MYCP) supplementation.
Conclusions
Acremonium coenophialum and lolii live in symbiosis with tall fescue and perennial ryegrass, respectively. The grass hosts provide the fungal symbionts with nutrition and reproductive viability, while the endophyte fungi protect their grass hosts from biotic and abiotic stresses. Concurrently, the endophytes produce mycotoxins that can be detrimental to the productivity of animals consuming the grasses. Ergovaline is the primary ergopeptine alkaloid associated with fescue toxicosis, but Nacetylloline and N-formylloline seem to contribute to the disorder. Lolitrem and paxilline are considered to be the main toxic agents in perennial ryegrass.
Although symptoms of these toxicities are readily and extensively defined, efforts to counteract them with a single treatment have been less successful. Specific non-performance indicators can show consistent symptomatic alleviation of toxicosis within narrow experimental windows. However, outside these windows, animal performance in practical grazing conditions is often not improved enough to be measured or to be aligned with performance predicted from the forage nutrient composition. The search must continue for plant/ animal management systems that will alleviate some, or all of the toxicity associated with these grasses. Currently, the yeast cell wall-based adsorbent shows promise in reducing toxin impact.
References
Ashley, T.L., D.G. Keltner, J.B. McLaren, H.A. Fribourg and D. Onks. 1987. Performance of crossbred cows and calves grazing fescue pastures with varying levels of Acremonium coenophialum infection. J. Anim. Sci. 65(Suppl. 1):49.
Bacon, C.W. 1994. Fungal endophytes, other fungi, and their metabolites as extrinsic factors of grass quality. In: Forage Quality, Evaluation, and Utilization (G.C. Fahey, Jr., ed), Amer. Soc. Agron., Crop Sci. Soc., Soil Sci. Soc., Madison, WI. p. 318.
Bacon, C.W., P.C. Lyons, J.L. Porter and J.D. Robbins. 1986. Ergot toxicity from endophyteinfected grasses: A review. Agron. J. 78:106.
Beers, K.W. and E.L. Piper. 1987. Effect of grazing endophyte-infected fescue on heifer growth, calving rate, and calf birth weight of first calf heifers. Arkansas Farm Res. 36(5):2.
Bluett, S.J., J. Hodgson, P.D. Kemp and T.N. Barry. 2001. Performance of lambs and the incidence of staggers and heat stress on two perennial ryegrass Lolium perenne cultivars using a leaderfollower rotational grazing management system. J. Agric. Sci. 136:99.
Bond, J., J.B. Powell and B.T. Weinland. 1984. Behavior of steers grazing various varieties of tall fescue during summer conditions. Agron. J. 76:707.
Bush, L.P. and R.C. Buckner. 1973. Tall fescue toxicity. In: Antiquality Components of Forages (A.G. Matches, ed), Crop Sci. Soc. Amer., Madison, WI. p. 99.
Bush, L.P., P.L. Cornelius, R.C. Buckner, D.R. Varney, R.A. Chapman, P.B. Burrus, C.W. Kennedy, T.A. Jones and M.J. Saunders. 1982. Association of N-acetylloline derivative and Nformylloline derivative with Epichloe typhina in tall fescue. Crop Sci. 22:941.
Bush, L.P., F.F. Fannin, M.R. Siegel, D.L. Dahlman and H.R. Burton. 1993. Chemistry, occurrence, and biological effects of saturated pyrrolizidine alkaloids associated with endophyte-grass interactions. Agric. Ecosyt. & Environ. 44:81.
Chestnut, A.B., P.B. Anderson, M.A. Cochran, H.A. Fribourg and K.D. Gwinn. 1992. Effects of hydrated sodium calcium aluminosilicate on fescue toxicosis and mineral absorption. J. Anim. Sci. 70:2838.
Clay, K. 1987. Effects of fungal endophytes on the seed and seedlings of Lolium perenne and Festuca arundinacea. Oecologia (Heidelb.) 73:358.
Clay, L. 1993. The ecology and evolution of endophytes. Agric. Ecosyst. & Environ. 44:39.
Coffey, K.P., J.L. Meyer, F.K. Brazle and L.W. Lomas. 1992. Amount and diurnal distribution of grazing time by stocker cattle under different tall fescue management strategies. Appl. Anim. Behav. Sci. 33:121.
Cole, N.A., J.A. Stuedemann, C.W. Purdy and D.P. Hutchinson. 1987. Influence of endophyte in tall fescue pastures on the feedlot performance of feeder steers. J. Anim. Sci. 65(Suppl. 1):331.
Crawford, R.J., J.R. Forwood, R.L. Belyea and G.B. Gardner. 1989. Relationship between level of endophyte infection and cattle gains on tall fescue. J. Prod. Agric. 2:147.
Danielson, D.A., S.P. Schmidt, C.C. King, L.A. Smith and W.B. Webster. 1986. Fescue toxicity and reproduction in beef heifers. J. Anim. Sci. 63(Suppl. 1):296.
Dawson, K.A. 1994. Manipulation of microorganisms in the digestive tract: The role of oligosaccharides and diet specific yeast cultures. Proc. California Anim. Nutr. Conf. for Feed Manufacturers.
DeBattista, J.P., J.H. Bouton, C.W. Bacon and M.R. Siegel. 1990. Rhizome and herbage production of endophyte-removed tall fescue clones and populations. Agron. J. 82:561.
Devegowda, G., B.I.R. Aravind and M.G. Morton. 1996. Saccharomyces cerevisiae and mannan oligosaccharides to counteract aflatoxicosis in broilers. Proc. Australian Poultry Sci. Symp., Sidney, Australia 8:103.
Ely, D.G., M.E. Benson, T.J. Smith and K.P. Coffey. 1985. Utilization of mefluidide treated Kentucky 31 tall fescue pastures by beef cows and calves. J. Anim. Sci. 61(Suppl. 1):335.
Evans, J. and K.A. Dawson. 2000. The ability of Mycosorb to bind toxins present in endophtyeinfected tall fescue. In: Biotechnology in the Feed Industry: Proceeding from Alltech’s 16th Annual Symposium (T.P. Lyons and K.A. Jacques, eds), Nottingham Press University, Nottingham, UK, pp. 409-422.
Forcherio, J.C., M.S. Kerley and J.A. Paterson. 1992. Performance of steers grazing tall fescue during the spring followed by warm-season grasses during the summer. J. Anim. Sci. 70(Suppl. 1):182.
Fribourg, H.A., A.B. Chestnut, R.W. Thompson, J.B. McLaren, R.J. Carlisle, K.D. Qwinn, M.C. Dixon and M.C. Smith. 1991. Steer performance in fescue clover pastures with different levels of endophyte infestation. Agron. J. 83:777.
Garner, G.B., R. Morrow, T. Fairbrother, J. Gerrish, C. Nelson, V. Jacobs and W. Hires. 1984. Nursing cows, calves, and steers response to simultaneously grazed tall fescue pastures with varying levels of endophyte E. typhina. In: Proc. Amer. Forage and Grassl. Conf., Houston, TX. Forage and Grassland Council, Lexington, KY.
Garner, G.B., G.E. Rottinghaus, C.N. Cornell and H. Testereci. 1993. Chemistry of compounds associated with endophyte/grass interactions: Ergovaline-and ergopeptine-related alkaloids. Agric. Ecosyst. & Environ. 44:65
Garrett, J.L., D.G. Ely, D.K. Aaron and J. Wyles. 1988. Mefluidide treatment of nitrogen fertilized Kentucky 31 tall-fescue pastures for yearling cattle. University of Kentucky, Beef Cattle Res. Rept. 306:40.
Gay, N., J.A. Boling, R. Dew and D.E. Miksch. 1988. Effects of endophyte-infected tall fescue on beef cow-calf performance. Appl. Agric. Res. 3:182.
Girard, I.D. 1996. Characterization of stimulatory activities from Saccharomyces cerevisiae on the growth and activities of ruminal bacteria. Ph.D. Dissertation, University of Kentucky, Lexington, KY.
Goetsch, A.L., A.L. Jones, K.W. Beers, S.R. Stokes and E.L. Piper. 1987. Effect of offering different amounts and types of supplemental feeds to growing dairy steers fed endophyte-infected fescue hay ad libitum on intake, digestion, passage rate, and serum prolactin concentration. J. Anim. Sci. 64:1769.
Hannah, S.M., J.A. Paterson, J.E. Williams, M.S. Kerley and J.L. Miner. 1990. Effects of increasing dietary levels of endophyte-infected tall fescue seed on digestibility and ruminal kinetics in sheep. J. Anim. Sci. 68:1693.
Hardy, T.N., K. Clay and A.M. Hammond, Jr. 1986. Leaf age and related factors affecting endophytemediated resistance to fall army worm (Lepitoptera:Noctuidae) in tall fescue. Environ. Entomol. 15:1083.
Hemken, R.W., L.S. Bull, J.A. Boling, E. Kane, L.P. Bush and R.C. Buckner. 1979. Summer fescue toxicosis in lactating dairy cows and sheep fed experimental strains of ryegrass-tall fescue hybrids. J. Anim. Sci. 49:641.
Hemken, R.W., J.A. Boling, L.S. Bull and R.H. Hatton. 1981. Interaction of environmental temperature and anti-quality factors on the severity of summer fescue toxicosis. J. Anim. Sci. 52:710.
Hemken, R.W., J.A. Jackson, Jr. and J.A. Boling. 1984. Toxic factors in tall fescue. J. Anim. Sci. 58:1011.
Hoveland, C.S., S.P. Schmidt, C.C. King, Jr. and E.M. Clark. 1984. Association of fungal endophyte with seasonal gains of beef steers grazing tall fescue pasture. In: Proc. European Grassl. Fed. (H. Riley and A.D. Skelvag, eds), pp. 382.
Hoveland, C. S. 1993. Economic importance of Acremonium endophytes. Agric. Ecosyst. & Environ. 44:3.
Howard, M.D., R.B. Muntifering, N.W. Bradley, G.E. Mitchell, Jr. and S.R. Lowry. 1992. Voluntary intake and ingestive behavior of steers grazing Johnstone or endophte-infected Kentucky 31 tall fescue. J. Anim. Sci. 70:1227.
Hurley, W.L., E.M. Corvey, K. Leung, L.A. Edgerton and R.W. Hemken. 1981. Bovine prolactin, TSH, T4, and T3 concentrations as affected by tall fescue summer toxicosis and temperature. J. Anim. Sci. 51:374.
Joost, R.E. 1995. Acremonium in tall fescue and ryegrass: Boon or bane? A review. J. Anim. Sci. 73:881.
Keltner, D.G., J.B. McLaren, H.A. Fribourg, A.B. Chestnut, W.T. McDonald and D.O. Onks. 1989. Performance of crossbred cows and calves grazing tall fescue with varying levels of endophyte infestation. J. Anim. Sci. 66(Suppl. 1):55.
Lusby, K.S., W.E. McMurphy, C.A. Strasia, S.C. Smith and S.H. Muntz. 1990. Effects of fescue endophyte and interseeded clovers on subsequent finishing performance of steers. J. Prod. Agric. 3:103.
Paterson, J., J.R. Forwood and K. Turner. 1987. University of Missouri report on fescue research activities. Proc. Tall Fescue Toxicosis Workshop, Memphis, TN.
Paterson, J., C. Forcherio, B. Larson, M. Samford and M. Kerley. 1995. The effects of fescue toxicosis on beef cattle productivity. J. Anim. Sci. 73:889.
Paul, R.M. 1999. The effects of artificial shade on tympanic temperature and production characteristics of grazing beef cattle. Ph.D. Dissertation, University of Kentucky, Lexington, KY.
Pederson, J.F., R. Rodriquez-Kabana and R.A. Shelby. 1988. Ryegrass cultivars and endophyte in tall fescue affect nematodes in grass and succeeding soybeans. Agron. J. 80:811.
Peters, C.W., K.N. Grigsby, C.G. Aldrich, J.A. Paterson, R.J. Lipsey, M.S. Kerley and G.B. Garner. 1992. Performance, forage utilization, and ergovaline consumption by beef cows grazing endophyte fungus-infected fescue, endophyte fungus-free fescue, or orchardgrass. J. Anim. Sci. 70:1550.
Pinkerton, B.S., J.S. Rice and D.J. Undersander. 1990. Germination in Festuca arundinacea as affected by the fungal endophyte Acremonium coenophialum. In: Proc. Int. Symp. Acremonium/Grass Interactions. Louisiana Agric. Exp. Sta., Baton Rouge, p. 176.
Porter, R.K. 1994. Chemical constituents of grass endophytes. In: Biotechnology of Endophytic Fungi of Grasses (C.W. Bacon and J.F. White, Jr., eds), Chap. 8. CRC Press, Boca Raton, FL.
Porter, R. K. 1995. Analysis of endophyte toxins: Fescue and other grasses toxic to livestock. J. Anim. Sci. 73:871.
Pratt, A.D. and J.L. Haynes. 1954. Herd performance on Kentucky-31 fescue. Ohio Farm Home Res. 35:10.
Prestidge, R.A. 1993. Causes and control of perennial ryegrass staggers in New Zealand. Agric. Ecosyst. & Environ. 44:283.
Prestidge, R.A., R.P. Pottinger and G.M. Barker. 1982. An association of Lolium endophyte with ryegrass resistance to Argentine stem weevil. In: Proc. New Zealand Weed Pest Control Conf., p. 119.
Prestidge, R.A., G.M. Barker and R.P. Pottinger. 1991. The economic cost of Argentine stem weevil in pastures in New Zealand. In: Proc. 44th New Zealand Weed Pest Control Conf., p. 165.
Prestidge, R.A., E.R. Thom, S.L. Marshall, M.J. Taylor, B. Willoughby and D.P. Wildermoth. 1992. Influence of Acremonium lolii infection in perennial ryegrass on germination emergence, survival and growth of white clover. New Zealand J. Agric. Res. 35:225.
Read, J.C. and B.J. Camp. 1986. The effect of the fungal endophyte Acremonium coenophialum in tall fescue on animal performance, toxicity, and stand maintenance. Agron. J. 78:848.
Rowan, D.D. 1993. Lolitrems, peramine, and paxilline: Mycotoxins of ryegrass/endophyte interaction. Agric. Ecosyst. & Environ. 44:103.
Schillo, K.K., L.S. Leshin, J.A. Boling and N. Gay. 1988. Effects of endophyte-infected fescue on concentrations of prolactin in blood sera and the anterior pituitary and concentratrions of dopamine and dopamine metabolites in brains of steers. J. Anim. Sci. 66:713.
Schmidt, S.P., D.A. Danielson, J.A. Holliman, H.W. Grimes and W.B. Webster. 1986. Fescue fungus suppresses growth and reproduction in replacement heifers. Alabama Agric. Exp. Sta. Highlights Agric. Res. 33(4):15.
Schmidt, S.P. and T.G. Osborn. 1993. Effects of endophyte-infected tall fescue on animal performance. Agric. Ecosyst. & Environ. 44:233.
Seman, D.H., J.A. Stuedemann, D.L. Breedlove, S.R. Wilkinson, D.P. Belesky, F.N. Thompson and F.P. Stewart. 1990. Difference in grazing behavior of steers consuming endophyte-infected or noninfected tall fescue. In: Proc. Int. Acremonium/Grass Interactions (S.S. Quisenberry and E. Joost, eds), Louisiana Agric. Exp. Sta., Baton Rouge, p. 267.
Stuedemann, J.A., T.S. Rumsey, J. Bond, S.R. Wilkinson, L.P. Bush, D.J. Williams and A.B. Caudle. 1985. Association of blood cholesterol with incidence of fat necrosis in cows and tall fescue summer toxicosis in steers. Amer. J. Vet. Res. 46:1990.
Stuedemann, J.A., D.L. Breedlove, K.R. Pond, D.P. Belesky, L.P. Tate, Jr., F.N. Thompson and S.R. Wilkinson. 1989. Effect of endophyte (Acremonium coenophialum) infection of tall fescue and paddock exchange on intake and performance of grazing steers. In: Proc. XVI Int. Grassl. Cong., Nice France, p. 1243.
Sutherland, B.L. and J.H. Hoglund. 1990. Effect of ryegrass containing the endophyte Acremonium lolii on associated white clover. In: Proc. Int. Symp. Acremonium/Grass Interactions (S.S. Quisenberry and R.E. Joost, eds), Louisiana Agric. Exp. Sta., Baton Rouge, p 67.
Welty, R.E., R.E. Barker and M.D. Azevedo. 1991. Reaction of tall fescue infected and noninfected by Acremonium coenophialum to Puccina graminis subsp. graminicola. Plant Dis. 75:883.
Yates, S.G., R.J. Petroski and R.G. Powell. 1990. Analysis of loline alkaloids in endophyte-infected fescue by capillary gas chromatography. J. Agric. Food Chem. 38:182.
Authors: D.G. ELY, D.K. AARON, S.E. BAR, V. AKAY and J.A. JACKSON
Department of Animal Sciences, University of Kentucky, Lexington, Kentucky, USA





.jpg&w=3840&q=75)