Contemporary perspectives on Fusarium mycotoxicoses in livestock and poultry
Published: June 14, 2007
By: TREVOR K. SMITH, H.V.L.N. SWAMY, SUSAN L. RAYMOND and MAHER ZAYTOUN (Courtesy of Alltech Inc.)
Mycotoxins are fungal metabolites that can reduce performance and alter metabolism of livestock and poultry (Wannemacher et al., 1991). The pathological states arising from the consumption of feeds contaminated with mycotoxins are termed mycotoxicoses.
Mycotoxins can be formed in the field preharvest and may continue to be formed under suboptimal storage conditions postharvest.
High moisture content often predisposes feedstuffs to fungal growth and mycotoxin formation.
Temperature is another key factor. Some fungi, such as Aspergillus flavus, are usually found in tropical and semi-tropical climates. This mold produces aflatoxin, a carcinogenic hepatotoxin. Fusarium fungi, however, are more common in temperate climates and Fusarium mycotoxins are likely the most common mycotoxins globally (Wood, 1992).
The global frequency of mycotoxin contamination of feedstuffs and the severity of mycotoxicoses in livestock and poultry appear to have increased in recent years. This may be due, in part, to increased monitoring of suspect materials and an increased awareness of the symptoms of mycotoxicoses by veterinarians and producers.
Global climate change has also contributed to an increased frequency of mycotoxin contamination of feed grains. Drought, excessive rainfall and flooding can all promote mold growth. Increased international trading of feedstuffs has also contributed to the problem as this increases the chance that a given compound feed will contain components of widely varying geographical origins. Such blends of ingredients increase the chance of the feed containing mixtures of different mycotoxins.
This can result in toxicological synergies that increase the severity of mycotoxicoses.
Symptoms typical of mycotoxicoses are often seen despite analysis of the feed that indicates only very low concentrations of individual mycotoxins (Trenholm et al., 1983). In this situation, it is not clear if a mycotoxin problem really exists, or if poor performance is due to management or nutritional factors.
It is now known that unexpected toxicity may be due to toxicological interactions between different mycotoxins that exaggerate the effects of any single compound. The likelihood of this occuring is greatest for the Fusarium mycotoxins.
It has been shown that consumption of naturally contaminated feedstuffs produces greater toxicity than does consumption of an equivalent amount of purified mycotoxins (Tremholm et al., 1994). It has also been shown that fusaric acid, the most common of the Fusarium mycotoxins (Bacon et al., 1996), can increase the toxicity of the trichothecene deoxynivalenol (vomitoxin, DON) (Smith et al., 1997). Fusaric acid, however, is seldom analyzed in feeds due to its low toxicity when consumed in the absence of other toxins (Smith and MacDonald, 1991; Smith and Sousadias, 1993).
The approach most commonly used to overcome mycotoxicoses in livestock and poultry is the use of specialty feed additives referred to as mycotoxin adsorbents (Ramos et al., 1996).
Hydrated sodium calcium aluminosilicate (HSCAS) has been shown to have potential to reduce aflatoxicosis but has been shown to not be effective against Fusarium toxicoses (Patterson and Young, 1993). Bentonite has been shown to be effective against T-2 toxin (Carson and Smith, 1983a) but only at levels that are not practical in animal feeds.
Other types of clays also have some potential benefits against T-2 toxin but again only at very high levels of dietary inclusion (Smith, 1984). Alfalfa fibre can have protective effects against zearalenone (James and Smith, 1982; Stangroom and Smith, 1984) and T-2 toxin (Carson and Smith, 1983b), but alfalfa is also often a source of Fusarium contamination in diets.
The problem of high levels of dietary inclusion has now been overcome with the development of polymeric extracts of yeast cell wall, which are the basis of the Mycosorb® product of Alltech, Inc.
Materials and methods
FEEDING TRIAL WITH HORSES
A study was conducted to determine the effect of feeding mature horses a blend of wheat and corn naturally contaminated with Fusarium mycotoxins.
Changes in intake of concentrate and blood metabolites and the efficacy of Mycosorb® to prevent these changes were determined. Nine nonexercising, mature mares were randomly assigned to one of three experimental concentrates for 21 days.
The experiment was subsequently replicated in time. Concentrates included: (1) control (2) contaminated grains (3) contaminated grains + 0.2% Mycosorb®. Concentrates containing contaminated grains contained about 12 ppm DON + about 20 ppm fusaric acid.
The total diets included 35% concentrates and 65% hay (5 kg/head/day). The hay and straw bedding were not contaminated with Fusarium mycotoxins. Concentrate intake, blood biochemical profiles and serum immunoglobulin concentrations were determined weekly.
FEEDING TRIALS WITH SWINE
Immunosuppression study
Starter pigs (initial weight approximately 9 kg) were fed five diets (7 pens of 5 pigs per diet) for three weeks. The diets included: (1) control (2) blend of wheat and corn naturally contaminated with Fusarium mycotoxins (3) contaminated grains + 0.05% Mycosorb® (4) contaminated grains + 0.1% Mycosorb® (5) contaminated grains + 0.2% Mycosorb®. Blood samples were taken after three weeks of feeding and serum was analyzed for immunoglobulin concentrations.
Pair feeding study
Starter pigs (initial weight approximately 9 kg) were fed five diets (7 pens of 5 pigs per diet) for three weeks. The diets included: (1) control (2) low level of contaminated grains (2.2 ppm DON + 36.22 ppm fusaric acid) (3) high level of contaminated grains (2.9 ppm DON + 49.28 ppm fusaric acid) (4) high level of contaminated grains + 0.2% Mycosorb® (2.8 ppm DON + 20.93 ppm fusaric acid) (5) pigs were pair-fed the control diet to the intake of pigs fed the high level of contaminated grains. Weight gains and feed intake were determined weekly. At the end of the experiment, jugular blood samples were drawn from all pigs for determination of serum metabolites.
FEEDING TRIALS WITH POULTRY
Immunosuppression study
A total of 360 broiler chicks of a commercial strain were fed four diets for 56 days (Swamy et al., 2002). The diets included: (1) control (2) low level of contaminated grains (4.7 ppm DON + 20.6 ppm fusaric acid + 0.2 ppm zearalenone) (3) high level of contaminated grains (8.2 ppm DON + 21.6 ppm fusaric acid + 0.56 ppm zearalenone) (4) high level of contaminated grains + 0.2% Mycosorb® (9.7 ppm DON + 21.6 ppm fusaric acid + 0.8m ppm zearalenone). Blood and biliary samples were collected after eight weeks and were analyzed for immunoglobulins.
Phytase study
A total of 252 day-old male broiler chicks were fed six experimental mash-type diets for three weeks (6 cages of 7 birds per diet). Phosphorus retention (phosphorus consumed minus phosphorus excreted) was determined over 3-day periods following weeks 1, 2 and 3. The diets included: (1) adequate phosphorus (2) phosphorus deficient (3) phosphorus deficient + phytase (4) phosphorus deficient + grains naturally contaminated with Fusarium mycotoxins (5) phosphorus deficient + phytase + contaminated grains (6) phosphorus deficient + phytase + contaminated grains + 0.2% Mycosorb®.
Results and discussion
FEEDING TRIAL WITH HORSES
Feeding contaminated grains to horses in the current study reduced concentrate intake compared to controls (P<0.05, Table 1). Supplementation with Mycosorb® to the blend of contaminated grains significantly improved concentrate intake compared to the feeding of contaminated grains. Consumption of forage remained unaffected regardless of diet fed.
Gamma-glutamyltransferase (GGT) levels were significantly higher in serum of horses consuming contaminated grain on days 7 and 14 but not on day 21, thereby implying that the horses might be adapting to the hepatotoxicity caused by the mixture of Fusarium mycotoxins. Feeding Mycosorb® prevented this hepatotoxicity.
Mycotoxins can be formed in the field preharvest and may continue to be formed under suboptimal storage conditions postharvest.
High moisture content often predisposes feedstuffs to fungal growth and mycotoxin formation.
Temperature is another key factor. Some fungi, such as Aspergillus flavus, are usually found in tropical and semi-tropical climates. This mold produces aflatoxin, a carcinogenic hepatotoxin. Fusarium fungi, however, are more common in temperate climates and Fusarium mycotoxins are likely the most common mycotoxins globally (Wood, 1992).
The global frequency of mycotoxin contamination of feedstuffs and the severity of mycotoxicoses in livestock and poultry appear to have increased in recent years. This may be due, in part, to increased monitoring of suspect materials and an increased awareness of the symptoms of mycotoxicoses by veterinarians and producers.
Global climate change has also contributed to an increased frequency of mycotoxin contamination of feed grains. Drought, excessive rainfall and flooding can all promote mold growth. Increased international trading of feedstuffs has also contributed to the problem as this increases the chance that a given compound feed will contain components of widely varying geographical origins. Such blends of ingredients increase the chance of the feed containing mixtures of different mycotoxins.
This can result in toxicological synergies that increase the severity of mycotoxicoses.
Symptoms typical of mycotoxicoses are often seen despite analysis of the feed that indicates only very low concentrations of individual mycotoxins (Trenholm et al., 1983). In this situation, it is not clear if a mycotoxin problem really exists, or if poor performance is due to management or nutritional factors.
It is now known that unexpected toxicity may be due to toxicological interactions between different mycotoxins that exaggerate the effects of any single compound. The likelihood of this occuring is greatest for the Fusarium mycotoxins.
It has been shown that consumption of naturally contaminated feedstuffs produces greater toxicity than does consumption of an equivalent amount of purified mycotoxins (Tremholm et al., 1994). It has also been shown that fusaric acid, the most common of the Fusarium mycotoxins (Bacon et al., 1996), can increase the toxicity of the trichothecene deoxynivalenol (vomitoxin, DON) (Smith et al., 1997). Fusaric acid, however, is seldom analyzed in feeds due to its low toxicity when consumed in the absence of other toxins (Smith and MacDonald, 1991; Smith and Sousadias, 1993).
The approach most commonly used to overcome mycotoxicoses in livestock and poultry is the use of specialty feed additives referred to as mycotoxin adsorbents (Ramos et al., 1996).
Hydrated sodium calcium aluminosilicate (HSCAS) has been shown to have potential to reduce aflatoxicosis but has been shown to not be effective against Fusarium toxicoses (Patterson and Young, 1993). Bentonite has been shown to be effective against T-2 toxin (Carson and Smith, 1983a) but only at levels that are not practical in animal feeds.
Other types of clays also have some potential benefits against T-2 toxin but again only at very high levels of dietary inclusion (Smith, 1984). Alfalfa fibre can have protective effects against zearalenone (James and Smith, 1982; Stangroom and Smith, 1984) and T-2 toxin (Carson and Smith, 1983b), but alfalfa is also often a source of Fusarium contamination in diets.
The problem of high levels of dietary inclusion has now been overcome with the development of polymeric extracts of yeast cell wall, which are the basis of the Mycosorb® product of Alltech, Inc.
Materials and methods
FEEDING TRIAL WITH HORSES
A study was conducted to determine the effect of feeding mature horses a blend of wheat and corn naturally contaminated with Fusarium mycotoxins.
Changes in intake of concentrate and blood metabolites and the efficacy of Mycosorb® to prevent these changes were determined. Nine nonexercising, mature mares were randomly assigned to one of three experimental concentrates for 21 days.
The experiment was subsequently replicated in time. Concentrates included: (1) control (2) contaminated grains (3) contaminated grains + 0.2% Mycosorb®. Concentrates containing contaminated grains contained about 12 ppm DON + about 20 ppm fusaric acid.
The total diets included 35% concentrates and 65% hay (5 kg/head/day). The hay and straw bedding were not contaminated with Fusarium mycotoxins. Concentrate intake, blood biochemical profiles and serum immunoglobulin concentrations were determined weekly.
FEEDING TRIALS WITH SWINE
Immunosuppression study
Starter pigs (initial weight approximately 9 kg) were fed five diets (7 pens of 5 pigs per diet) for three weeks. The diets included: (1) control (2) blend of wheat and corn naturally contaminated with Fusarium mycotoxins (3) contaminated grains + 0.05% Mycosorb® (4) contaminated grains + 0.1% Mycosorb® (5) contaminated grains + 0.2% Mycosorb®. Blood samples were taken after three weeks of feeding and serum was analyzed for immunoglobulin concentrations.
Pair feeding study
Starter pigs (initial weight approximately 9 kg) were fed five diets (7 pens of 5 pigs per diet) for three weeks. The diets included: (1) control (2) low level of contaminated grains (2.2 ppm DON + 36.22 ppm fusaric acid) (3) high level of contaminated grains (2.9 ppm DON + 49.28 ppm fusaric acid) (4) high level of contaminated grains + 0.2% Mycosorb® (2.8 ppm DON + 20.93 ppm fusaric acid) (5) pigs were pair-fed the control diet to the intake of pigs fed the high level of contaminated grains. Weight gains and feed intake were determined weekly. At the end of the experiment, jugular blood samples were drawn from all pigs for determination of serum metabolites.
FEEDING TRIALS WITH POULTRY
Immunosuppression study
A total of 360 broiler chicks of a commercial strain were fed four diets for 56 days (Swamy et al., 2002). The diets included: (1) control (2) low level of contaminated grains (4.7 ppm DON + 20.6 ppm fusaric acid + 0.2 ppm zearalenone) (3) high level of contaminated grains (8.2 ppm DON + 21.6 ppm fusaric acid + 0.56 ppm zearalenone) (4) high level of contaminated grains + 0.2% Mycosorb® (9.7 ppm DON + 21.6 ppm fusaric acid + 0.8m ppm zearalenone). Blood and biliary samples were collected after eight weeks and were analyzed for immunoglobulins.
Phytase study
A total of 252 day-old male broiler chicks were fed six experimental mash-type diets for three weeks (6 cages of 7 birds per diet). Phosphorus retention (phosphorus consumed minus phosphorus excreted) was determined over 3-day periods following weeks 1, 2 and 3. The diets included: (1) adequate phosphorus (2) phosphorus deficient (3) phosphorus deficient + phytase (4) phosphorus deficient + grains naturally contaminated with Fusarium mycotoxins (5) phosphorus deficient + phytase + contaminated grains (6) phosphorus deficient + phytase + contaminated grains + 0.2% Mycosorb®.
Results and discussion
FEEDING TRIAL WITH HORSES
Feeding contaminated grains to horses in the current study reduced concentrate intake compared to controls (P<0.05, Table 1). Supplementation with Mycosorb® to the blend of contaminated grains significantly improved concentrate intake compared to the feeding of contaminated grains. Consumption of forage remained unaffected regardless of diet fed.
Gamma-glutamyltransferase (GGT) levels were significantly higher in serum of horses consuming contaminated grain on days 7 and 14 but not on day 21, thereby implying that the horses might be adapting to the hepatotoxicity caused by the mixture of Fusarium mycotoxins. Feeding Mycosorb® prevented this hepatotoxicity.
Table 1. Effect of feeding blends of grains naturally contaminated with Fusarium mycotoxins on feed intake and hepatic metabolism of horses.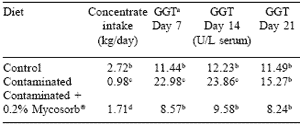 aγ-glutamyltransferase activity. b,c,d Means in a column differ (P<0.05). |
It has been reported that horses are quite resistant to the effects of DON-contaminated barley (Johnson et al., 1997). Feeding 1.27 kg of barley contaminated with 36-44 ppm DON per day for 40 days did not affect feed intake or immunological status of horses.
It is likely that the feeding of a blend of contaminated grains in the current study resulted in combinations of mycotoxins and toxicological synergies (Smith et al., 1997) that were not present in the study of Johnson et al. (1997).
It was concluded that supplementation of yeast cell wall polymer (Mycosorb®) to diets naturally contaminated with Fusarium mycotoxins was beneficial in alleviating reduced feed intake and metabolic changes in mature horses.
FEEDING TRIALS WITH SWINE
Immunosuppression study
The effect of feeding grains naturally contaminated with Fusarium mycotoxins on serum immunoglobulin concentrations in starter pigs is given in Table 2.
Feeding grains naturally contaminated with Fusarium mycotoxins increased serum concentrations of immunoglobulin M (P<0.037) and immunoglobulin A (IgA) (P<0.01) although concentrations of immunoglobulin G (IgG) were not affected by diet. The contaminated grain-induced increases in IgA and IgG were reduced in a quadratic manner by feeding increasing levels of Mycosorb® (P<0.024 and P<0.006 respectively).
It has been reported that the feeding of DON to mice results in increased serum concentarations of immunoglobulins M and G (Forsell et al., 1986).
The pigs are responding in a similar manner although the combinations of mycotoxins fed in the current study differ from the feeding of an individual toxin in the case of Forsell et al. (1986). It can be concluded that the immunosuppressive effects arising from feeding combinations of Fusarium mycotoxins can be overcome by the dietary inclusion of Mycosorb®.
Table 2.Effect of feeding blends of grains naturally contaminated with Fusarium mycotoxins on serum immunoglobulin concentrations (mg/ml) in starter pigs.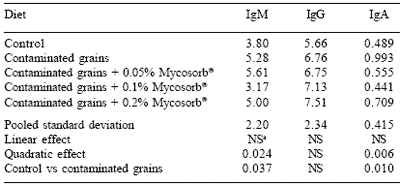 aNot significant (P>0.05). |
Table 4.Effect of feeding blends of grains naturally contaminated with Fusarium mycotoxins on serum metabolites of starter pigs (0-21 days). To enlarge the image, click here aNot significant (P>0.05). |
Pair feeding study
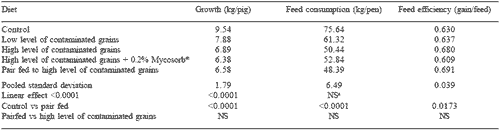
The effect of feeding blends of grains naturally contaminated with Fusarium mycotoxins on weight gain, feed consumption and feed efficiency of starter pigs is given in Table 3. There was a significant linear decline in weight gain and feed consumption with the feeding of increasing levels of contaminated grains. Feed efficiency was largely unaffected by diet.
The effect of diet on serum metabolites is given in Table 4. It was observed that pigs pair-fed to the feed intake of those consuming the high level of contaminated grains had significantly higher concentrations of total serum proteins and globulins. This infers that the adverse effects of feeding grains naturally contaminated with Fusarium mycotoxins are due not only to appetite suppression but also to metabolic changes. It is of note that the feeding of Mycosorb® prevented these changes.
Serum concentrations of conjugated bilirubin are an index of hunger. This was reflected in the observation that serum conjugated bilirubin concentrations were significantly elevated in pairfed pigs compared to controls (P<0.0134). The toxic effects on feed intake resulting from the high mycotoxin concentrations used in the current study were too great to be overcome by the feeding of 0.2% Mycosorb®.
FEEDING TRIALS WITH POULTRY
Immunosuppression study
The effect of feeding grains naturally contaminated with Fusarium mycotoxins on biliary immunoglobulin concentrations in finisher chickens at eight weeks of age is given in Table 5.
Feeding of grains naturally-contaminated with Fusarium mycotoxins resulted in no significant effect on serum concentrations of immunoglobulins A, G or M (P>0.05) but there was a significant decrease in biliary IgM concentrations. This decrease was prevented by feeding 0.2% Mycosorb®. This immunoglobulin is particularly important in protecting the alimentary and respiratory tracts from infection.
It is clear that the immune system of broilers is responding differently to the feeding of grains contaminated with Fusarium mycotoxins when compared to pigs and mice. It can be concluded, however, that as with pigs, the effects of Fusarium mycotoxins on the broiler immune system can be overcome by the feeding of Mycosorb®.
Phytase study
The effect of feeding grains naturally contaminated with Fusarium mycotoxins on the efficacy of exogenous phytase is given in Table 6. Feeding grains containing Fusarium mycotoxins significantly increased phosphorus retention compared to the feeding of a phosphorous deficient diet (P<0.05).
This effect was reduced when the contaminated grains were also supplemented with 0.02% Mycosorb®.
Table 5. Effect of feeding blends of grains naturally contaminated with Fusarium mycotoxins on biliary IgA concentrations in finisher broilers. a,bMeans within a column differ (P<0.05). |
Feeding trichothecene mycotoxins causes lesions of the intestinal tract and is characterized by hemorrhaging, ulcers and bloody scours (Wannemacher et al., 1991). Such pathology is likely to reduce nutrient uptake and might increase phosphorus excretion when exogenous phytase is fed.
The current study was conducted to test this hypothesis. Phosphorus retention was increased, however, with the feeding of contaminated grain; and this was not affected by inclusion of exogenous phytase. It was clear that feeding contaminated grains did not reduce the benefits of feeding phytase.
It is possible that Fusarium fungal spores and/or mycotoxins may also contain phytase-like activity.
The spores should be viable since the feed was not pelleted. The effect of feeding contaminated grains on phosphorus retention was not significant when Mycosorb® was included in the diet, inferring that the mycotoxins themselves might be promoting phosphorus uptake, although the mechanism by which this might occur is not clear.
| Conclusions It can be concluded that the adverse effects of Fusarium mycotoxins in a wide range of animal species can be overcome by feeding the yeast cell wall-based adsorbent. This has important economic consequences when widespread contamination of the feed supply forces use of contaminated grains or when favorable pricing prompts the intentional feeding of contaminated materials. |
Table 6.Effect of feeding blends of grains naturally contaminated with Fusarium mycotoxins on phosphorus retention of chicks fed exogenous phytase with and without Mycosorb®. a,b,c Means in a column differ (P<0.05). |
References
Bacon, C.W., J.K. Porter, W.P. Norred and J.F. Leslie. 1996. Production of fusaric acid by Fusarium species. Appl. Environ. Microbiol. 62:4039.
Carson, M.S. and T.K. Smith. 1983a. Role of bentonite in the prevention of T-2 toxicosis in rats. J. Anim. Sci. 57:1498.
Carson, M.S. and T.K. Smith. 1983b. Effect of feeding alfalfa and refined plant fibres on the toxicity and metabolism of T-2 toxin in rats. J. Nutr. 113:304.
Forsell, J.H., M.F. Witt, J.H. Tai, R. Jensen and J.J. Pestka. 1986. Effects of 8-week exposure of the B6C3F1 mouse to dietary deoxynivalenol (vomitoxin) and zearalenone. Food Chem Toxicol. 24(3):213-219.
James, L.J. and T.K. Smith. 1982. Effect of dietary alfalfa on zearalenone toxicity and metabolism in rats and swine. J. Anim. Sci. 55:110.
Johnson, P.J., S.W. Casteei, and N.T. Messer. 1997. Effect of feeding deoxynivalenol (vomitoxin)- contaminated barley to horses. J. Vet. Diagn. Invest. 9:219.
Patterson, R. and L.G. Young. 1993. Efficacy of hydrated sodium calcium aluminosilicate, screening and dilution in reducing the effects of mold contaminated corn in pigs. Can. J. Anim. Sci. 73:615.
Ramos, A.-J., J. Fink-Gremmels and E. Hernandez. 1996. Prevention of toxic effects of mycotoxins by means of non-nutritive adsorbent compounds. J. Food Protection 59:631.
Smith, T.K. 1984. Spent canola oil bleaching clays: potential for treatment of T-2 toxicosis in rats and short-term inclusion in diets for immature swine. Can. J. Anim. Sci. 64:725.
Smith, T.K. and E.J. MacDonald. 1991. Effect of fusaric acid on brain regional neurochemistry and vomiting behavior in swine. J. Anim. Sci. 69:2044.
Smith, T.K., E.G. McMillan and J.B. Castillo. 1997. Effect of feeding blends of Fusarium mycotoxincontaminated grains containing deoxynivalenol and fusaric acid on growth and feed consumption of immature swine. J. Anim. Sci. 75:2184.
Smith, T.K. and M.G. Sousadias. 1993. Fusaric acid content of swine feedstuffs. J. Agric. Food Chem. 41:2296.
Stangroom, K.E. and T.K. Smith. 1984. Effect of whole and fractionated dietary alfalfa meal on zearalenone toxicosis in rats and swine. Can. J. Physiol. Pharmacol. 62:1219.
Swamy, H.V.L.N., T.K. Smith, P.F. Cotter, H.J. Boermans and A.E. Sefton. 2002. Effects of feeding blends of grains naturally-contaminated with Fusarium mycotoxins on production and metabolism in broilers. Poult. Sci. 84: in press.
Trenholm, H.L., W.P. Cochrane, H.Cohen, J.I. Elliott, E.R. Farnworth, D.W. Friend, R.M.G. Hamilton, J.R. Standish and B.K. Thompson. 1983. Survey of vomitoxin contamination of 1980 Ontario winter wheat crop: Results of survey and feeding trials. J. Assoc. Offic. Anal. Chem. 66:92.
Trenholm, H.L., B.C. Foster, L.L. Charmley, B.K. Thompson, K.E. Hartin, R.W. Coppock and M.A. Albassam. 1994. Effects of feeding diets containing Fusarium (naturally) contaminated wheat or pure deoxynivalenol (DON) in growing pigs. Can. J. Anim. Sci. 74:361.
Wannemacher, R.W., D.L. Bunner and H.A. Neufeld. 1991. Toxicity of trichothecenes and other related mycotoxins in laboratory animals. In: Mycotoxins and Animal Feeds (J.E. Smith and R.S. Henderson, eds) CRC Press, Boca Raton, FL.
Wood, G.E. 1992. Mycotoxins in foods and feeds in the United States. J. Anim. Sci. 70:3941.
Authors: TREVOR K. SMITH 1, H.V.L.N. SWAMY 1, SUSAN L. RAYMOND 2 and MAHER ZAYTOUN 1
1 Department of Animal and Poultry Science, University of Guelph, Guelph, Ontario, Canada.
2 Equine Research Centre, Guelph, Ontario, Canada.
Related topics
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.







.jpg&w=3840&q=75)