Chitoneous Materials for Control of Foodborne Pathogens and Mycotoxins in Poultry
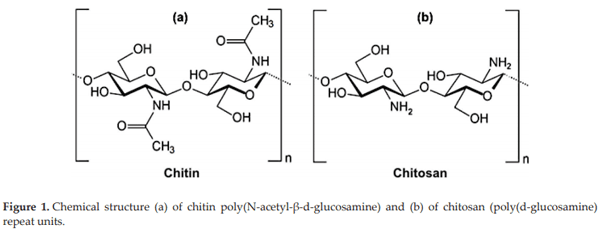
Public concern with the incidence of antibiotic-resistant bacteria, particularly among foodborne pathogens has been challenging the poultry industry to find alternative means of control. Chitosan is a modified, natural biopolymer derived by deacetylation of chitin. The antimicrobial activity and film-forming property of chitosan makes it a potential source of food preservative or coating material of natural origin for improvement of quality and shelf life of various foods of agriculture, poultry, beef and seafood origin. In addition to its use as an antimicrobial, it has been shown that it has good properties as a mycotoxin adsorbent. The purposes of the present chapter is to summarize our experience using chitin-chitosan from Deacetylated 95% food grade chitosan (Paragon Specialty Products LLC Rainsville, AL) or Aspergillus oryzae meal (Fermacto®, PetAg Inc., Hampshire IL) to control foodborne pathogens, improve performance, biological sanitizer and mycotoxin binder in commercial poultry.
Keywords: chitosan, Fermacto®, Salmonella, mycotoxins, gut health
1. Introducción
Bornet A, Teissedre PL. Chitosan, chitin-glucan and chitin effects on minerals (iron, lead, cadmium) and organic (ochratoxin A) contaminants in wines. European Food Research and Technology. 2008;226:681-689. DOI: 10.1007/s00217-007-0577-0
Szymczyk P, Filipkowska U, Józwiak T, Kuczajowska-Zadrozna M. Phosphate removal from aqueous solutions by chitin and chitosan in flakes. Progress on Chemistry and Application of Chitin and its Derivatives. 2016;21:260-272
Rinaudo M. Chitin and chitosan: Properties and applications. Progress in Polymer Science. 2006;31:603-632
Ahmed TA, Aljaeid BM. Preparation, characterization, and potential application of chi- tosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Design, Development and Therapy. 2016;10:483
Kumar MNVR. A review of chitin and chitosan applications. Reactive and Functional Polymers. 2000;46:1-27
Senel S, McClure SJ. Potential applications of chitosan in veterinary medicine. Advanced Drug Delivery Reviews. 2004;56:1467-1480
Kong M, Chen XG, Xing K, Park HJ. Antimicrobial properties of chitosan and mode of action: A state of the art review. International Journal of Food Microbiology. 2010;144:51-63
Helander IM, Nurmiaho-Lassila E-L, Ahvenainen R, Rhoades J, Roller S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. International Journal of Food Microbiology. 2001;71:235-244
Chung Y-C, Su YP, Chen C-C, Jia G, Wang HL, Wu JCG, Lin JG. Relationship between anti- bacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacologica Sinica. 2004;25:932-936
Filipkowska U, Józwiak T, Szymczyk P. Application of cross-linked chitosan for phos- phate removal from aqueous solutions. Progress on Chemistry and Application of Chitin and its Derivatives. 2014;19:5-14
Luo Y, Wang Q. Recent advances of chitosan and its derivatives for novel applications in food science. Journal of Food Processing & Beverages. 2013;1:1-13
Rao SB, Sharma CP. Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. Journal of Biomedical Materials Research. 1997;34:21-28
Muzzarelli RAA. Chitins and chitosans as immunoadjuvants and non-allergenic drug carriers. Marine Drugs. 2010;8:292-312
Divya K, Vijayan S, George TK, Jisha MS. Antimicrobial properties of chitosan nanopar- ticles: Mode of action and factors affecting activity. Fibers and Polymers. 2017;18:221-230
Romainor ANB, Chin SF, Pang SC, Bilung LM. Preparation and characterization of chitosan nanoparticles-doped cellulose films with antimicrobial property. Journal of Nanomaterials. 2014;2014:130
Boyle EC, Bishop JL, Grassl GA, Finlay BB. Salmonella: From pathogenesis to therapeu- tics. Journal of Bacteriology. 2007;189:1489-1495
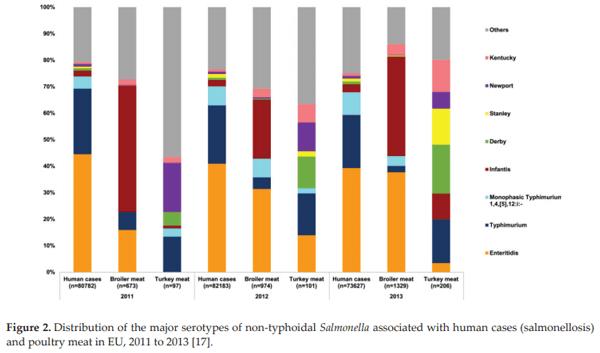
Antunes P, Mourão J, Campos J, Peixe L. Salmonellosis: The role of poultry meat. Clinical Microbiology and Infection. 2016;22:110-121
Naksuriya O, Okonogi S. Comparison and combination effects on antioxidant power of curcumin with gallic acid, ascorbic acid, and xanthone. Drug Discoveries & Therapeutics. 2015;9:136-141
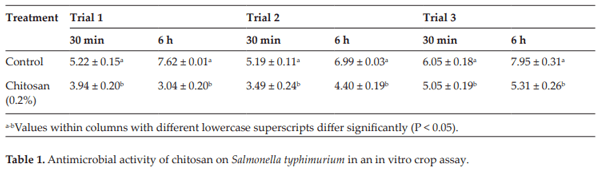
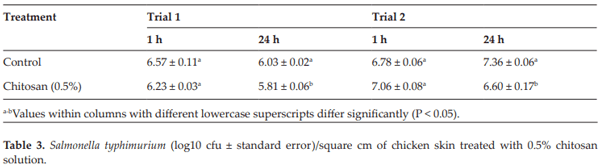
Menconi A, Hernandez-Velasco X, Latorre JD, Kallapura G, Pumford NR, Morgan MJ, Hargis BM, Tellez G. Effect of chitosan as a biological sanitizer for Salmonella typhimurium and aerobic Gram negative spoilage bacteria present on chicken skin. International Journal of Poultry Science. 2013;12:318
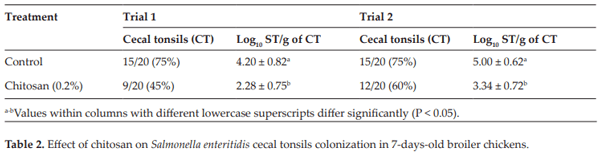
Menconi A, Pumford NR, Morgan MJ, Bielke LR, Kallapura G, Latorre JD, Wolfenden AD, Hernandez-Velasco X, Hargis BM, Tellez G. Effect of chitosan on Salmonella typhimurium in broiler chickens. Foodborne Pathogens and Disease. 2014;11:165-169
Corrier DE, Byrd JA, Hargis BM, Hume ME, Bailey RH, Stanker LH. Presence of Salmonella in the crop and ceca of broiler chickens before and after preslaughter feed withdrawal. Poultry Science. 1999;78:45-49
Byrd JA, Hargis BM, Caldwell DJ, Bailey RH, Herron KL, McReynolds JL, Brewer RL, Anderson RC, Bischoff KM, Callaway TR. Effect of lactic acid administration in the drinking water during preslaughter feed withdrawal on Salmonella and Campylobacter contamination of broilers. Poultry Science. 2001;80:278-283
Cox JM, Pavic A. Advances in enteropathogen control in poultry production. Journal of Applied Microbiology. 2010;108:745-755
Choi EH, Yang HP, Chun HS. Chitooligosaccharide ameliorates diet-induced obesity in mice and affects adipose gene expression involved in adipogenesis and inflammation. Nutrition Research. 2012;32:218-228
Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. Journal of Controlled Release. 2004;100:5-28
Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. The Journal of Nutrition. 1995;125:1401
Liu X, Cao S, Zhang X. Modulation of gut microbiota–brain axis by probiotics, prebiot- ics, and diet. Journal of Agricultural and Food Chemistry. 2015;63:7885-7895
Torres-Rodriguez A, Sartor C, Higgins SE, Wolfenden AD, Bielke LR, Pixley CM, Sutton L, Tellez G, Hargis BM. Effect of Aspergillus meal prebiotic (Fermacto) on performance of broiler chickens in the starter phase and fed low protein diets. Journal of Applied Poultry Research. 2005;14:665-669
Mizutani O, Shiina M, Yoshimi A, Sano M, Watanabe T, Yamagata Y, Nakajima T, Gomi K, Abe K. Substantial decrease in cell wall α-1, 3-glucan caused by disruption of the kexB gene encoding a subtilisin-like processing protease in Aspergillus oryzae. Bioscience, Biotechnology, and Biochemistry. 2016;80:1781-1791
Guio F, Rugeles LD, Rojas SE, Palomino MP, Camargo MC, Sánchez OF. Kinetic modeling of fructooligosaccharide production using Aspergillus oryzae N74. Applied Biochemistry and Biotechnology. 2012;167:142-163
Bays HE, Evans JL, Maki KC, Evans M, Maquet V, Cooper R, Anderson JW. Chitin- glucan fiber effects on oxidized low-density lipoprotein: A randomized controlled trial. European Journal of Clinical Nutrition. 2013;67:2-7
Uchima CA, Tokuda G, Watanabe H, Kitamoto K, Arioka M. Heterologous expression and characterization of a glucose-stimulated β-glucosidase from the termite Neotermes koshu- nensis in Aspergillus oryzae. Applied Microbiology and Biotechnology. 2011;89:1761-1771
Cox E, Verdonck F, Vanrompay D, Goddeeris B. Adjuvants modulating mucosal immune responses or directing systemic responses towards the mucosa. Veterinary Research. 2006;37:511-539
Corrigan A, Horgan K, Clipson N, Murphy RA. Effect of dietary supplementation with a Saccharomyces cerevisiae mannan oligosaccharide on the bacterial community structure of broiler cecal contents. Applied and Environmental Microbiology. 2011;77:6653-6662
Janssens GP j, Millet S, Van Immerseel F, De Buck J, Hesta M. The impact of prebiotics and sal monellosis on apparent nutrient digestibility and Salmonella typhimurium var. Copenhagen excretion in adult pigeons (Columba livia domestica). Poultry Science. 2004;83:1884-1890
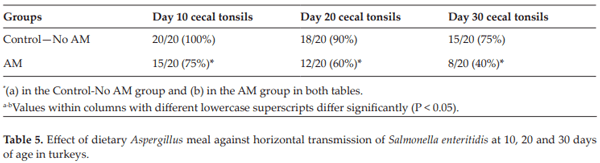
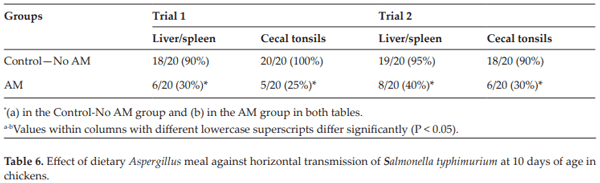
Londero A, Menconi A, Reginatto AR, Bacocina AI, Wolfenden A, Shivaramaiah S, Hargis BM, Tellez G. Effect of an Aspergillus meal prebiotic on salmonella infection in turkeys and broiler chickens. International Journal of Poultry Science. 2011;10:946-951
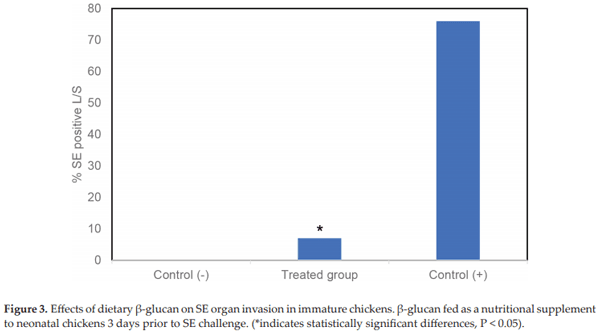
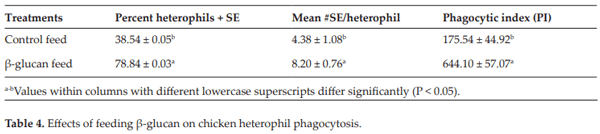
Lowry VK, Farnell MB, Ferro PJ, Swaggerty CL, Bahl A, Kogut MH. Purified β-glucan as an abiotic feed additive up-regulates the innate immune response in immature chickens against Salmonella enterica serovar Enteritidis. International Journal of Food Microbiology. 2005;98:309-318
Xu ZR, Hu CH, Xia MS, Zhan XA, Wang MQ. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poultry Science. 2003;82:1030-1036
Dahiya JP, Wilkie DC, Van Kessel AG, Drew MD. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Animal Feed Science and Technology. 2006;129:60-88
Kim HG, Lee SY, Kim NR, Lee HY, Ko MY, Jung BJ, Kim CM, Lee JM, Park JH, Han SH. Lactobacillus plantarum lipoteichoic acid down-regulated Shigella flexneri peptidoglycan- induced inflammation. Molecular Immunology. 2011;48:382-391
Huang RL, Yin YL, Wu GY, Zhang YG, Li TJ, Li LL, Li MX, Tang ZR, Zhang J, Wang B. Effect of dietary oligochitosan supplementation on ileal digestibility of nutrients and performance in broilers. Poultry Science. 2005;84:1383-1388
Merino-Guzmán R, Latorre JD, Delgado R, Hernandez-Velasco X, Wolfenden AD, Teague KD, Graham LE, Mahaffey BD, Baxter MFA, Hargis BM. Comparison of total immunoglobulin A levels in different samples in leghorn and broiler chickens. Asian Pacific Journal of Tropical Biomedicine. 2017;7:116-120
Beal RK, Wigley P, Powers C, Hulme SD, Barrow PA, Smith AL. Age at primary infec- tion with Salmonella enterica serovar Typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Veterinary Immunology and Immunopathology. 2004;100:151-164
Harms RH, Miles RD. Research note: Influence of Fermacto® on the performance of laying hens when fed diets with different levels of methionine. Poultry Science. 1988;67:842-844
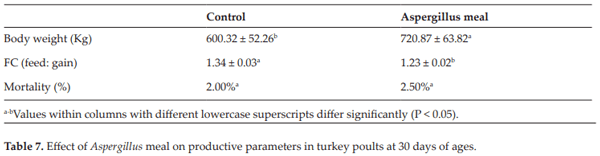
Tellez G, Nava GM, Vincente JL, De Franceschi M, Morales EJ, Prado O, Hargis BM. Evaluation of dietary Aspergillus meal on intestinal morphometry in turkey poults. International Journal of Poultry Science. 2010;9:75-878
Reginatto AR, Menconi A, Londero A, Lovato M, Rosa AP, Shivaramaiah S, Wolfenden AD, Huff WE, Huff GR, Rath NC. Effects of dietary Aspergillus meal prebiotic on tur- key poults production parameters and bone qualities. International Journal of Poultry Science. 2011;10:496-499
Uni Z, Ganot S, Sklan D. Posthatch development of mucosal function in the broiler small intestine. Poultry Science. 1998;77:75-82
Tellez G, Higgins SE, Donoghue AM, Hargis BM. Digestive physiology and the role of microorganisms. Journal of Applied Poultry Research. 2006;15:136-144
Latorre JD, Hernandez-Velasco X, Kuttappan VA, Wolfenden RE, Vicente JL, Wolfenden AD, Bielke LR, Prado-Rebolledo OF, Morales E, Hargis BM. Selection of Bacillus spp. for cellulase and xylanase production as direct-fed microbials to reduce digesta viscosity and Clostridium perfringens proliferation using an in vitro digestive model in different poultry diets. Frontiers in Veterinary Science. 2015;2:25
Latorre JD, Hernandez-Velasco X, Wolfenden RE, Vicente JL, Wolfenden AD, Menconi A, Bielke LR, Hargis BM, Tellez G. Evaluation and selection of Bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as direct-fed microbial candidates for poultry. Frontiers in Veterinary Science. 2016;3:95
Latorre JD, Hernandez-Velasco X, Vicente JL, Wolfenden R, Hargis BM, Tellez G. Effects of the inclusion of a Bacillus direct-fed microbial on performance parameters, bone quality, recovered gut microflora, and intestinal morphology in broilers consuming a grower diet containing corn distillers dried grains with solubles. Poultry Science. 2017;96:2728-2735
Hussein HS, Brasel JM. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology. 2001;167:101-134
Zain ME. Impact of mycotoxins on humans and animals. Journal of Saudi Chemical Society. 2011;15:129-144
Armando MR, Pizzolitto RP, Dogi CA, Cristofolini A, Merkis C, Poloni V, Dalcero AM, Cavaglieri LR. Adsorption of ochratoxin A and zearalenone by potential probi- otic Saccharomyces cerevisiae strains and its relation with cell wall thickness. Journal of Applied Microbiology. 2012;113:256-264
Streit E, Schatzmayr G, Tassis P, Tzika E, Marin D, Taranu I, Tabuc C, Nicolau A, Aprodu I, Puel O. Current situation of mycotoxin contamination and co-occurrence in animal feed—Focus on Europe. Toxins (Basel). 2012;4:788-809
Smith LE, Stoltzfus RJ, Prendergast A. Food chain mycotoxin exposure, gut health, and impaired growth: A conceptual framework. Advances in Nutrition: An International Review. 2012;3:526-531
Greco MV, Franchi ML, Rico Golba SL, Pardo AG, Pose GN. Mycotoxins and mycotoxigenic fungi in poultry feed for food-producing animals. Scientific World Journal. 2014;2014:1-9
Galarza-Seeber R, Latorre JD, Bielke LR, Kuttappan VA, Wolfenden AD, Hernandez- Velasco X, Merino-Guzman R, Vicente JL, Donoghue A, Cross D. Leaky gut and mycotoxins: Aflatoxin B1 does not increase gut permeability in broiler chickens. Frontiers in Veterinary Science. 2016;3:10
Jouany JP. Methods for preventing, decontaminating and minimizing the toxicity of mycotoxins in feeds. Animal Feed Science and Technology. 2007;137:342-362
Kolosova A, Stroka J. Evaluation of the effect of mycotoxin binders in animal feed on the analytical performance of standardised methods for the determination of mycotoxins in feed. Food Additives & Contaminants: Part A. 2012;29:1959-1971
Avantaggiato G, Solfrizzo M, Visconti A. Recent advances on the use of adsorbent mate- rials for detoxification of Fusarium mycotoxins. Food Additives and Contaminants. 2005;22:379-388
Di Natale F, Gallo M, Nigro R. Adsorbents selection for aflatoxins removal in bovine milks. Journal of Food Engineering. 2009;95:186-191
Hokkanen S, Bhatnagar A, Sillanpää M. A review on modification methods to cellulose- based adsorbents to improve adsorption capacity. Water Research. 2016;91:156-173
Tan KB, Abdullah AZ, Horri BA, Salamatinia B. Adsorption mechanism of microcrystal- line cellulose as green adsorbent for the removal of cationic methylene blue dye. Journal of the Chemical Society of Pakistan. 2016;38:651-664
Zhao L, Yang G, Shi Y, Su C, Chang J. Co-delivery of Gefitinib and chloroquine by chitosan nanoparticles for overcoming the drug acquired resistance. Journal of Nano- biotechnology. 2015;13:57. DOI: 10.1186/s12951-015-0121-5
Mine Kurtbay H, Bekçi Z, Merdivan M, Yurdakoç K. Reduction of ochratoxin A levels in red wine by bentonite, modified bentonites, and chitosan. Journal of Agricultural and Food Chemistry. 2008;56:2541-2545
Ledoux DR, Rottinghaus GE. In vitro and in vivo testing of adsorbents for detoxifying mycotoxins in contaminated feedstuffs. Biotechnology Feed Industry. Nottingham, UK: Nottingham University Press; 1999. pp. 369-379
Kong C, Shin SY, Kim BG. Evaluation of mycotoxin sequestering agents for aflatoxin and deoxynivalenol: An in vitro approach. Spring. 2014;3:346
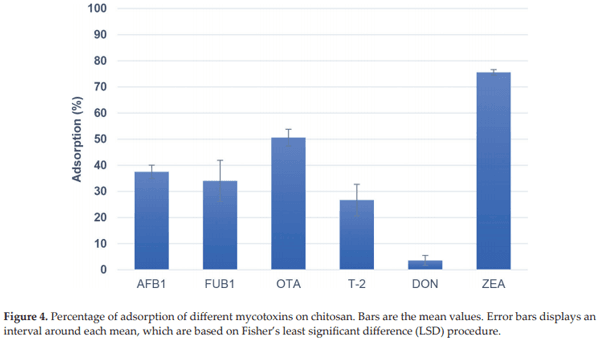
Solís-Cruz B, Hernández-Patlán D, Beyssac E, Latorre JD, Hernandez-Velasco X, Merino- Guzman R, Tellez G, López-Arellano R. Evaluation of chitosan and cellulosic polymers as binding adsorbent materials to prevent aflatoxin B1, fumonisin B1, ochratoxin, tricho- thecene, deoxynivalenol, and zearalenone mycotoxicoses through an in vitro gastroin- testinal model for poultry. Polymers (Basel). 2017;9:529
Zhao Z, Liu N, Yang L, Wang J, Song S, Nie D, Yang X, Hou J, Wu A. Cross-linked chito- san polymers as generic adsorbents for simultaneous adsorption of multiple mycotox- ins. Food Control. 2015;57:362-369
Dakovic A, Tomaševic-Canovic M, Rottinghaus GE, Matijaševic S, Sekulic Ž. Fumonisin B 1 adsorption to octadecyldimethylbenzyl ammonium-modified clinoptilolite-rich zeo- litic tuff. Microporous and Mesoporous Materials. 2007;105:285-290
Bazin I, Faucet-Marquis V, Monje M-C, El Khoury M, Marty J-L, Pfohl-Leszkowicz A. Impact of pH on the stability and the cross-reactivity of ochratoxin A and citrinin. Toxins (Basel). 2013;5:2324-2340
Ghadi A, Mahjoub S, Tabandeh F, Talebnia F. Synthesis and optimization of chitosan nanoparticles: Potential applications in nanomedicine and biomedical engineering. Caspian Journal of Internal Medicine. 2014;5:156
Khan RU, Naz S, Javdani M, Nikousefat Z, Selvaggi M, Tufarelli V, Laudadio V. The use of turmeric (Curcuma longa) in poultry feed. World’s Poultry Science Journal. 2012;68:97-103
Khalafalla RE, Müller U, Shahiduzzaman M, Dyachenko V, Desouky AY, Alber G, Daugschies A. Effects of curcumin (diferuloylmethane) on Eimeria tenella sporozoites in vitro. Parasitology Research. 2011;108:879-886
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: Problems and promises. Molecular Pharmaceutics. 2007;4:807-818
Hernandez-Patlan D, Solis-Cruz B, Méndez-Albores A, Latorre JD, Hernandez-Velasco X, Tellez G, López-Arellano R. Comparison of PrestoBlue® and plating method to evalu- ate antimicrobial activity of ascorbic acid, boric acid and curcumin in an in vitro gas- trointestinal model. Journal of Applied Microbiology. 2017;124:423-430. DOI: 10.1111/ jam.13659
Rivera-Rodriguez GR, Lollo G, Montier T, Benoit JP, Passirani C, Alonso MJ, Torres D. In vivo evaluation of poly-l-asparagine nanocapsules as carriers for anti-cancer drug delivery. International Journal of Pharmaceutics. 2013;458:83-89. DOI: 10.1016/j. ijpharm.2013.09.038
Alishahi A, Mirvaghefi A, Tehrani MR, Farahmand H, Shojaosadati SA, Dorkoosh FA, Elsabee MZ. Shelf life and delivery enhancement of vitamin C using chitosan nanopar- ticles. Food Chemistry. 2011;126:935-940
Luo Y, Teng Z, Wang Q. Development of zein nanoparticles coated with carboxymethyl chitosan for encapsulation and controlled release of vitamin D3. Journal of Agricultural and Food Chemistry. 2012;60:836-843
Dimzon IKD, Ebert J, Knepper TP. The interaction of chitosan and olive oil: Effects of degree of deacetylation and degree of polymerization. Carbohydrate Polymers. 2013;92:564-570
Oyarzun-Ampuero FA, Rivera-Rodríguez GR, Alonso MJ, Torres D. Hyaluronan nanocap- sules as a new vehicle for intracellular drug delivery. European Journal of Pharmaceutical Sciences. 2013;49:483-490
Rokhati N, Widjajanti P, Pramudono B, Susanto H. Performance comparison of α- and β-amylases on chitosan hydrolysis. ISRN Chemical Engineering. 2013;2013:186159
Ma H, Qi X, Maitani Y, Nagai T. Preparation and characterization of superparamagnetic iron oxide nanoparticles stabilized by alginate. International Journal of Pharmaceutics. 2007;333:177-186. DOI: 10.1016/j.ijpharm.2006.10.006
Déat-Lainé E, Hoffart V, Garrait G, Beyssac E. Whey protein and alginate hydrogel mic- roparticles for insulin intestinal absorption: Evaluation of permeability enhancement properties on Caco-2 cells. International Journal of Pharmaceutics. 2013;453:336-342
Vázquez-Durán A, Díaz-Torres R, Ramírez-Noguera P, Moreno-Martínez E, Méndez- Albores A. Cytotoxic and genotoxic evaluation of tortillas produced by microwave heat- ing during alkaline-cooking of aflatoxin-contaminated maize. Journal of Food Science. 2014;79:T1024-T1029
Schipper NG, Varum KM, Artursson P. Chitosans as absorption enhancers for poorly absorbable drugs. 1: Influence of molecular weight and degree of acetylation on drug transport across human intestinal epithelial (Caco-2) cells. Pharmaceutical Research. 1996;13:1686-1692
Prego C, Torres D, Fernandez-Megia E, Novoa-Carballal R, Quiñoá E, Alonso MJ. Chitosan–PEG nanocapsules as new carriers for oral peptide delivery: Effect of chitosan pegylation degree. Journal of Controlled Release. 2006;111:299-308
Xiang Y, Liu Y, Mi B, Leng Y. Hydrated polyamide membrane and its interaction with algi- nate: A molecular dynamics study. Langmuir. 2013;29:11600-11608. DOI: 10.1021/la401442r
Van der Lubben IM, Verhoef JC, Borchard G, Junginger HE. Chitosan and its derivatives in mucosal drug and vaccine delivery. European Journal of Pharmaceutical Sciences. 2001;14:201-207. DOI: 10.1016/S0928-0987(01)00172-5
Amidi M, Mastrobattista E, Jiskoot W, Hennink WE. Chitosan-based delivery systems for protein therapeutics and antigens. Advanced Drug Delivery Reviews. 2010;62:59-82. DOI: 10.1016/j.addr.2009.11.009
Salatin S, Yari Khosroushahi A. Overviews on the cellular uptake mechanism of polysaccharide colloidal nanoparticles. Journal of Cellular and Molecular Medicine. 2017;21:1668-1686. DOI: 10.1111/jcmm.13110
Li Q, Liu C-G, Yu Y. Separation of monodisperse alginate nanoparticles and effect of particle size on transport of vitamin E. Carbohydrate Polymers. 2015;124:274-279. DOI: 10.1016/j.carbpol.2015.02.007
The information and research on the use of Chitosan and its derivatives in Pharma and in Preserving the feeds of poultry, cattle, etc. is a highlight. To control E coli, Salmonella, Candida like other harmful bacteria, there are Herbal, Aromatic oils and Phyto products
in Nature. One should find each herb or oil which control the particular harmful bacteria.
For the Bioavailability of the product Pepper, Pippali and dry Ginger are to be used in micro levels in Feeds. The ultimate use of the herbal consumption will develop Immune system in Birds and Animals leading to good health and less expenditure on drugs.










.jpg&w=3840&q=75)