Associated efficiency of Saccharomyces cerevisiae and vitamin E in ameliorating adverse effects of ochratoxin A on production performance in broiler chickens
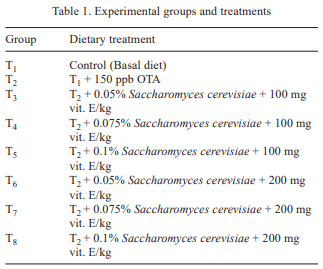
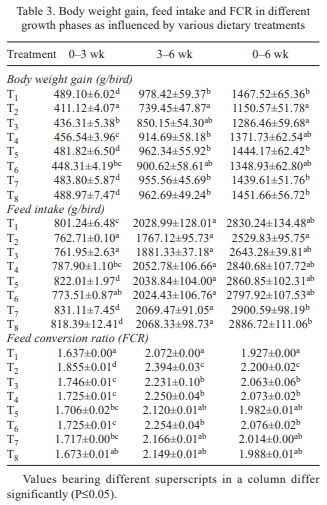
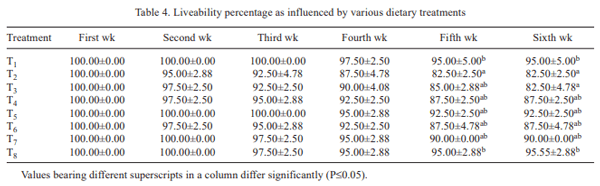
In the present study, efficiency of Saccharomyces cerevisiae and vitamin E together in ameliorating ochratoxicosis in broiler chickens was investigated. Day-old broiler chicks (320) were divided into 8 treatment groups (T1- control (basal diet); T2- T1+ 150 ppb OTA; T3-T2 + 0.05% SC + 100 mg vitamin E-VE; T4- T2 + 0.075% SC + 100 mg VE; T5- T2 + 0.1% SC + 100 mg VE; T6- T2 + 0.05% SC + 200 mg VE; T7- T2 + 0.075% SC + 200 mg VE; T8- T2 + 0.1% SC + 200 mg VE per kg diet). Each diet was fed to 5 replicated groups of 8 birds from 0 to 42 days of age. During overall growth period (0–6 weeks), the body weight gain (BWG) of birds fed ochratoxin contaminated diet (T2) was lower than that of control group (T1). The BWG of group T5, T7 and T8 was higher than T2 but statistically similar to that of control. During overall growth period, the FI in control group was statistically similar to other treatment groups. The FI in groups T7 and T8 was higher than that of group received basal diet with toxin (T2). The overall FCR in control group (T1) was lower than that of T2. The FCR in groups T3, T4 and T6 was higher than the control, but lower than that of T2. The FCR in groups T5, T7 and T8 was lower than T2 and statistically similar to that of control (T1). The overall liveability percentage in control group (T1) was higher than that of ochratoxin fed group (T2). The liveability percentage in group T3 was lower than control and similar to that of T2. The liveability percentage in groups T4 to T8 was statistically similar to that of control. Ochratoxin contamination in diet caused significant reduction in body weight gain, feed consumption, feed efficiency and livability percentage. It was concluded that inclusion of S. cerevisiae at 0.1% level along with 100 mg vitamin E per kg diet or S. cerevisiae at 0.075% level along with 200 mg vitamin E/kg diet to the ochratoxin (150 ppb) contaminated feed ameliorated the adverse effects of ochratoxicosis on production performance of broiler chickens.
Key words: Broiler chicken, Ochratoxin, Saccharomyces cerevisiae, Vitamin E.
Agawane S B and Lonkar P S. 2004.Effect of probiotic containing Saccharomyces boulardiion experimental ochratoxicosis in broilers: hematobiochemical studies. Journal of Veterinary Science 5: 359–67.
AOAC. 1995. Official Methods of Analysis. 15th edn. Association of Official Analytical Chemists, Washington, DC.
Baudrimont I, Berbeder A M, Charbi A, Pfohl-Leszkowicz A, Dirheimer G and Creppy E E. 1994. Effect of superoxide dismutase and catalase on the nephrotoxicity induced by subchronical administration of ochratoxinA in rats. Toxicology 89: 101–11.
Belmadani A, Steyn P S, Tramu G, Betbeder A M, Baudrimont I and Creppy E E. 1999. Selective toxicity of ochratoxinA in primary cultures from different brain regions. Archives of Toxicology 73: 108–14.
Denli M, Blandon J C, Guynot M E, Salado S and Perez J F. 2008. Efficacy of a new ochratoxin-binding agent OcraTox to counteract the deleterious effects of ochratoxin A in laying hens. Poultry Science 87: 2266–72.
Devegowda G, Raju M V L N, Afzali N and Swamy H V L N. 1998. Mycotoxin picture worldwide: Novel solutions for their counteraction. Feed Compounder 18: 22–27.
Elaroussi M A, Mohamed F R, El-Barkouky E M, Atta A M, Abdou A M and Hatab M H. 2006. Experimental ochratoxicosis in broiler chickens. Avian Pathology 4: 263–69.
El-Barkouky E M, Mohamed F R, Atta A M, Abu-Taleb A M, ElMenawey M A and Hatab M H. 2010. Effect of Saccharomyces cerevisiae and vitamin C supplementation on broiler performance subjected to ochratoxin A contamination. Egyptian Poultry Science Journal 30: 89–113.
El-Barkouky E M. 2008. The role of yeast in improving the performance of male broiler chicken fed ration contaminated with ochratoxin. Egyptian Journal of Applied Science 23: 13– 24.
El-Barkouky E M and Abu-Taleb A M. 2008. The role of vitamin C in improving the performance of male broiler chickens fed ration contaminated with ochratoxin. Egyptian Journal of Applied Science 23: 1–12.
Garcia A R, Avila E, Rosiles R and Petrone V M. 2003. Evaluation of two mycotoxin binders to reduce toxicity of broiler diets containing ochratoxin A and T–2 toxin contaminated grain. Avian Diseases 47: 691–99.
Hanif N Q, Muhammad G, Siddique M, Khanum A, Ahmed T, Gadahai J A and Kaukab G. 2008. Clinico-pathomorphological, serum biochemical and histological studies in broilers -fed ochratoxin A and a toxin deactivator Mycofix Plus. British Poultry Science 49: 632–42.
Koynarski V, Stoev S, Grozeva N, Mirtcheva T, Daskalo H, Mitev J and Mantle P. 2007. Experimental coccidiosis provoked by Eimeria acervulina in chicks simultaneously fed on ochratoxin A contaminated diet. Research in Veterinary Science 82: 225– 31.
Kubena L F, Harvey R B, Huff W E, Corrier D E, Philips T D and Creger CR. 1988. Influence of ochratoxin A and deoxynivalenol on growing broiler chicks. Poultry Science 67: 253–60.
Kubena L F, Harvey R B, Huff W E, Corrier D E, Philips T D and Rottinghaus G E. 1989. Influence of ochratoxin A and T-2 toxin singly and in combination on broiler chickens. Poultry Science 68: 867–72.
Kubena L F, Philips T D, Creger C R, Witzel D A and Heidelbaugh N D. 1983. Toxicity of ochratoxin A and tannic acid to growing chicks. Poultry Science 62: 1786–92.
Mahesh B K and Devegowda G. 1996. Ability of aflatoxin binders to bind aflatoxin in contaminated poultry feeds. An in vitro study. Proceedings of the 20th World’s Poultry Congress, New Delhi, India. pp 296–303.
Monnet-Tschudi F, Sorg O, Honegger P, Zurich M G, Huggett A C and Schilter B. 1997. Effects of the naturally occurring food Mycotoxin ochratoxin A on brain cells in culture. Neurotoxicology 18: 831–39.
Nahrer K and Kovalsky P. 2014. The biominmycotoxin survey identifying the threats in 2013. Science and Solutions 22: 2– 7.
Omar R F, Hasinoff B B, Mejilla F and Rahimtula A D. 1990. Mechanism of ochratoxin A stimulated lipid peroxidation. Biochemical Pharmacology 40: 1183–91.
Rahimtula A D, Bereziat J C, Bussacchini-Griot V and Bartsch H. 1988. Lipid peroxidation as a possible cause of ochratoxin A toxicity. Biochemical Pharmacology 37: 4469–77.
Sakhare P S, Harne S D, Kalorey D R, Warke S R, Bhandarkar A G and Kurkure N V. 2007. Effect of Toxiroak® polyherbal feed supplement during induced aflatoxicosis, ochratoxicosis and combined mycotoxicoses in broilers. Veterinarski Arhiv 77: 129–46.
Santin E, Paulillo A C, Nakagui L S O, Alessi A C and Maiorka A. 2006. Evaluation of yeast cell wall on the performance of broilers fed diets with or without mycotoxins. Brazilian Journal of Poultry Science 8: 221–22.
Sawale G K, Gosh R C, Ravikanth K, Maini S and Rekhe D S. 2009. Experimental mycotoxicosis in layer induced by ochratoxin A and its amelioration with herbomineral toxin binder toxiroak. International Journal of Poultry Science 8: 798–803.
Shiraishi F, Curtis L M, Truong L, Poss K, Visner G A, Madsen K, Nick H S and Agarwal A. 2000. Heme oxygenase–1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. American Journal of Physiology Renal Physiology 278: F726–36.
Singh M, Singh R and Mandal A B. 2016. Use of Saccharomyces cerevisiae to suppress the effects of ochratoxicosis in broiler chickens. Indian Journal of Animal Sciences 86: 790–94.
Singh M. 2015. ‘Influence of dietary Saccharomyces cerevisiae and vitamin E supplementation on ochratotoxicosis in broiler chickens’. M.V.Sc. Thesis, IVRI, Izatnagar, Uttar Pradesh.
Singh R, Mandal A B, Sharma M and Biswas A. 2015. Effect of varying levels of dietary ochratoxin A on the performance of broiler chickens. Indian Journal of Animal Sciences 85: 296– 300.
Singh R, Tyagi P K, Divya and Sharma M. 2013. Ochratoxigenic potential of Aspergillus westerdijkiae NRRL 3174 under laboratory conditions. Indian Journal of Poultry Science 48: 247–49.
Sorrenti V, Giacomo C D, Acquaviva R, Barbagallo I, Bognanno M and Galvano F. 2013. Toxicity of ochratoxin A and its modulation by antioxidants: A review. Toxins (Basel) 5: 1742– 66.
Stanley V G, Ojo R, Woldesenbet S, Hutchinson D H and Kubena L F. 1993.The use of Saccharomyces cerevisiae to suppress the effects of aflatoxicosis in broiler chicks. Poultry Science 72: 1867–72.
Stoev S D, Steanov M, Denev S, Radic B, Domijan A M and Peraica M. 2004. Experimental mycotoxicosis in chickens induced by ochratoxin A and penicillic acid and intervention with natural plant extracts. Veterinary Research Communication 28: 727–46.
Talapatra S K, Ray S C and Sen K C. 1940. Estimation of phosphorus, choline, calcium, magnesium, sodium and potassium in feeding stuffs. Journal of Veterinary Science and Animal Husbandary 10: 243–45.
Varga J and Toth B. 2005. Novel strategies to control mycotoxins in feeds: A review. Acta Veterinari Hungarica 53:189–203.
Verma J, Johri T S, Swain B K and Ameena S. 2004. Effect of graded levels of aflatoxin, ochratoxin and their combinations on the performance and immune response of broilers. British Poultry Science 45: 512–18.
Volkl A and Karlovsky P. 1998. Biological detoxification of fungal toxins and its use in plant breeding and feed and food production. Natural Toxins 7: 1–23.








