Aspergillus derived mycotoxins in food and the environment: Prevalence, detection, and toxicity
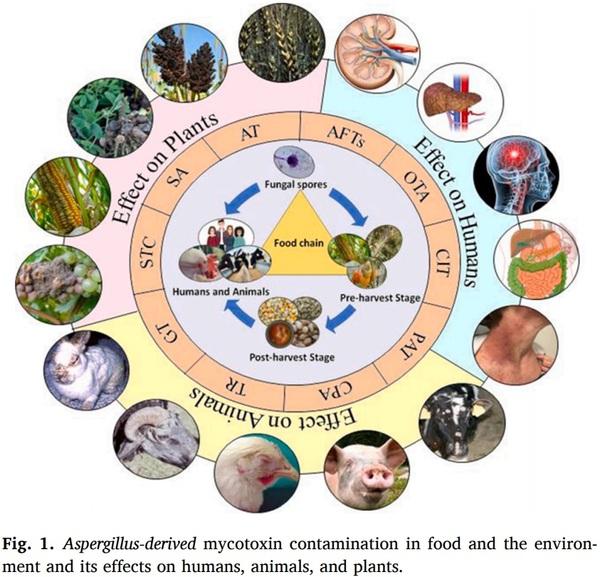
Aspergillus species are the paramount ubiquitous fungi that contaminate various food substrates and produce biochemicals known as mycotoxins. Aflatoxins (AFTs), ochratoxin A (OTA), patulin (PAT), citrinin (CIT), aflatrem (AT), secalonic acids (SA), cyclopiazonic acid (CPA), terrein (TR), sterigmatocystin (ST) and gliotoxin (GT), and other toxins produced by species of Aspergillus plays a major role in food and human health. Mycotoxins exhibited wide range of toxicity to the humans and animal models even at nanomolar (nM) concentration. Consumption of detrimental mycotoxins adulterated foodstuffs affects human and animal health even trace amounts. Bioaerosols consisting of spores and hyphal fragments are active elicitors of bronchial irritation and allergy, and challenging to the public health. Aspergillus is the furthermost predominant environmental contaminant unswervingly defile lives with a 40–90 % mortality risk in patients with conceded immunity. Genomics, proteomics, transcriptomics, and metabolomics approaches useful for mycotoxins’ detection which are expensive. Antibody based detection of toxins chemotypes may result in cross-reactivity and uncertainty. Aptamers (APT) are single stranded DNA (ssDNA/RNA), are specifically binds to the target molecules can be generated by systematic evolution of ligands through exponential enrichment (SELEX). APT are fast, sensitive, simple, in-expensive, and field-deployable rapid point of care (POC) detection of toxins, and a better alternative to antibodies.
Keywords: Aspergillus species, Mycotoxins, Foodstuffs, Environment, OMICS, Aptamers, Point of care (PoC).
[1] V. Koteswara Rao, P. Shilpa, S. Girisham, S.M. Reddy, Incidence of mycotoxigenic penicillia in feeds of Andhra Pradesh, India, Int. J. Biotechnol. Mol. Biol. Res. 2 (2016) 46–50.
[2] FAO (Food and Agriculture Organization), Worldwide Regulations for
Mycotoxins in Food and Feed in 2003, Information Division, FAO, Rome, 2003.
[3] Y. Suanthie, M.A. Cousin, C.P. Woloshuk, Multiplex real-time PCR for detection and quantification of mycotoxigenic Aspergillus, Penicillium and Fusarium,
J. Stored Prod. Res. 45 (2008) 139–145.
[4] R.G. Ruben, A.R. Soares, F. Corrado, C. Domenico, C. Giampiero, C. Virginia,
M. Raquel Aires-Barros, Advances, challenges and opportunities for point-ofneed screening of mycotoxins in foods and feeds, Analyst 143 (2018) 1015–1035.
[5] J.W. Bennett, M. Klich, Mycotoxins, Clin. Microbiol. Rev. 16 (3) (2003) 497–516.
[6] J.L. Baker, P. Bayman, N.E. Mahoney, M.A. Klich, J.D. Palumbo, B.C. Campbell,
Ochratoxigenic A. lanosusand A. alliaceus isolates from California tree nut orchards, in: Proceedings of the 3rd Fungal Genomics, 4th Fumonisin, and 16th
Aflatoxin Elimination Workshops, Savannah, Georgia, 2003.
[7] Z. R´ aduly, L. Szabo, ´ A. Madar, I. Pocsi, ´ L. Csernoch, Toxicological and medical aspects of aspergillus-derived mycotoxins entering the feed and food chain, Front.
Microbiol. 10 (2020) 2908.
[8] RASFF (The rapid alert system for food and feed) Annual report, European communities implications of mycotoxin in animal feed, Pak. J. Nature 5 (2008)
398–403.
[9] T.E. Cleveland, D. Bhatnagar, Molecular regulation of aflatoxin biosynthesis, in:
G.A. Bray, D.H. Ryan (Eds.), Mycotoxins, Cancer and Health, Vol. 1, Pennington
Center Nutrition Series, LSU Press, Baton Rouge, La, 1991, pp. 270–287.
[10] P.C. Bhandari, B. Bhandari, Aflatoxin and indian childhood cirrhosis, Indian
Pediatr. 17 (1980) 593–596.
[11] C. Gruber-Dorninger, T. Jenkins, G. Schatzmayr, Global mycotoxin occurrence in feed: a ten-year survey, Toxins (Basel) 11 (2019) 375.
[12] R.E. Minto, C.A. Townsend, Enzymology and molecular biology of aflatoxin biosynthesis, Chem. Rev. 97 (1997) 2537–2556.
[13] A.A.M. Safwan, N. Bacha, T. Alharazi, Detection of total aflatoxins in groundnut and soybean samples in Yemen using enzyme-linked immunosorbent assay,
J. Food Qual. 1 (2019) 7.
[14] G.G. Moore, B.M. Mack, S.B. Beltz, O. Puel, Genome sequence of an aflatoxigenic pathogen of Argentinian peanut, Aspergillus arachidicola, BMC Genomics 19 (2018) 1.
[15] A.O. El-zupir, A.S. Alamer, M.F. Dutton, The occurrence of aflatoxin in rice worldwide: a review, Toxin 34 (2015) 37–42.
[16] O. Ozluoymak, E. Guzel, Prediction of aflatoxin contamination on dried fig Ficus
Carica samples by spectral image analysis in comparison with laboratory results,
Fresenius Environ. Bull. 27 (2018).
[17] P. Andrade, E. Caldas, Aflatoxins in cereals: worldwide occurrence and dietary risk assessment, World Mycotoxin J. 8 (4) (2015) 415–431.
[18] J. Makhlouf, A. Carvajal-Campos, A. Querin, S. Tadrist, O. Puel, S. Lorber,
Morphologic, molecular and metabolic characterization ofAspergillus section flavi in spices marketed in Lebanon, Sci. Rep. 9 (2019) 1.
[19] B. Keliani, M.M. Sawada, C.E.D.C. Rodrigues, C.R.D. Fonseca, C.A.F. Oliveira,
Incidence of aflatoxins in oil seeds and possible transfer to oil: a review, Food
Environ. Rev. 6 (2014) 20–28.
[20] P.N. Pires, E.A. Vargas, M.B. Gomes, C.B.M. Vieira, E.A.D. Santos, A.A.C. Bicalho,
S.C. Silva, et al., Aflatoxins and ochratoxin a: occurrence and contamination levels in cocoa beans from Brazil, Food Addit. Contam. 36 (2019) 815–824.
[21] S. Okoth, Improving the Evidence Base on Aflatoxin Contamination and Exposure in Africa, CTA Working Paper, 2016.
[22] W.P. Blount, Turkey “X” disease, J. Brit. Turkey Fedr. 9 (1961) 55–58.
[23] B.F. Nesbitt, J. Okelly, K. Sargeant, A. Sheridan, Aspergillus flavus and Turkey X disease: toxic metabolites of Aspergillus flavus, Nature 195 (1962) 1062–1063.
[24] A. Ngindu, P. Kenya, D. Ocheng, T. Omondi, W. Ngare, D. Gatei, An outbreak of acute hepatitis caused by aflatoxin poisoning in Kenya, Lancet 319 (1982)
1346–1348.
[25] T. Asao, G. Buchi, M.M. Abdel-Kader, S.B. Chang, L.W. Emily, G.N. Wogan,
Aflatoxins B and G, J. Am. Chem. Soc. 85 (11) (1963) 1706–1707.
[26] J.M. Cullen, B.H. Ruebner, L.S. Hsieh, D.M. Hyde, D.P. Hsieh, Carcinogenicity of dietary aflatoxin M1 in male fischer rats compared to aflatoxin B1, Cancer Res. 47 (1987) 1913–1917.
[27] R.O. Sinnhuber, D. Lee, J.H. Wales, M.K. Landers, A.C. Keyl, Hepatic carcinogenesis of aflatoxin M1 in rainbow trout Salmo gairdneri and its enhancement by cyclopropene fatty acids, JNCI (1974) 1285–1288.
[28] F. Galvano, V. Galofaro, G. Galvano, Occurrence and stability of aflatoxin M1 in milk and milk products: a worldwide review, J. Food Prot. 59 (1996) 1079–1090.
[29] K.M. Amanda, R.L. Stephen, Mechanisms underlying aflatoxin-associated mutagenesis–implications in carcinogenesis, DNA Repair 77 (2019) 76–86.
[30] Y.M. Abdulrazzaq, R. Padmanabhan, S. Bastaki, J. Kochyil, M. Shafiullah,
Teratogenic effects of aflatoxin B1 in mice exposed in early and late gestation,
Pediatr. Res. 70 (2011), 405–405.
[31] B.R. Rushing, M.I. Selim, Aflatoxin B1: a review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods, Food
Chem. Toxicol. 124 (2019) 81–100.
[32] M.E. Zain, Impact of mycotoxins on humans and animals, J. Saudi Chem. Soc. 15 (2011) 129–144.
[33] T.O. Oluwaseun, N.E. Chibundu, K.E. Mari, S. Bojan, D. Babalola, S. Michael,
H. Jana, E. Christopher, K. Rudolf, Mycotoxin co-exposures in infants and young children consuming household- and industrially-processed complementary foods in Nigeria and risk management advice, Food Control 98 (2019) 312–322.
[34] L.W. Leslie, P.A. Egner, C.L. Belanger, R. Wattanawaraporn, L.J. Trudel, R.
G. Croy, J.D. Groopman, Aflatoxin B1-DNA adduct formation and mutagenicity in livers of neonatal male and female B6C3F1, Med. Toxicol. Sci. 122 (2011) 38–44.
[35] N.W. Gerald, T.W. Kensler, J.D. Groopman, Present and future directions of translational research on aflatoxin and hepatocellular carcinoma. A review, Food
Addtive Cont. Part A 29 (2012) 249–257.
[36] E.K. Kim, D.H. Shon, J.Y. Yoo, D. Ryu, C. Lee, Y.B. Kim, Natural occurrence of aflatoxins in korean meju, Food Addtive Cont. 18 (2001) 151–156.
[37] E.S. Maryann, S.S. Currier, E.A. Bailey, J.M. Essigmann, The chemistry and biology of aflatoxin B1: from mutational spectrometry to carcinogenesis,
Carcinogenesis 22 (2001) 535–545.
[38] Z. Xubo, D.W. Schaffner, T. Yue, Quantification of aflatoxin risk associated with chinese spices: point and probability risk assessments for aflatoxin B1, Food
Control 33 (2013) 366–377.
[39] B. Yohannes, W. Ayalew, A. Getachew, Analysis to ascertain the determination for aflatoxin contamination of milk and feeds from Gurage Zone, Ethiopia, Int. J.
Agric. Res. 13 (2018) 1–11.
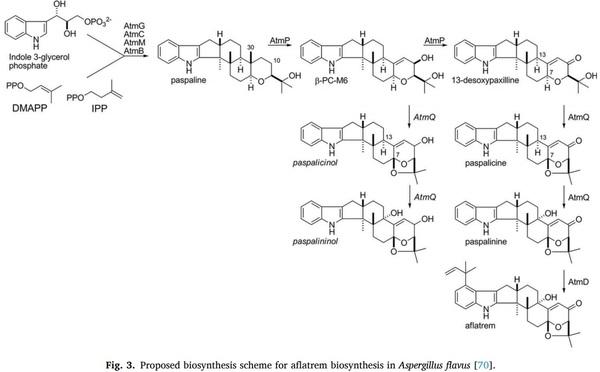
[40] G. Kathleen, J.P. Chinnici, G.C. M.Lewellyn, Effects of aflatoxin B1, aflatoxin B2, aflatoxin G1, and sterigmatocystin on viability, rates of development, and body length in two strains of Drosophila melanogaster Diptera, J. Invertebrate Pathol.
39 (1982) 388–394.
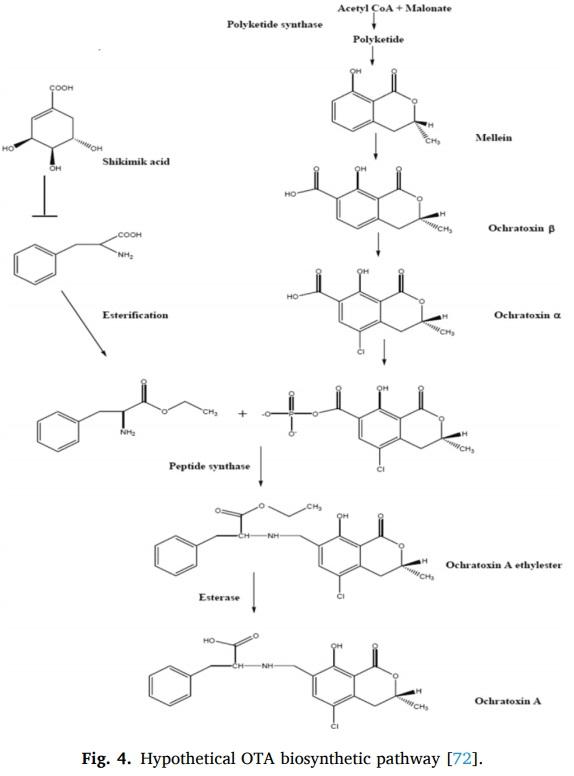
[41] Y. Takahashi, N. Yoko, Z. Shaomin, S. Yasuhito, K. Haruki, T. Kiyoji, Fumio Ide,
Enhanced spontaneous and aflatoxin-induced liver tumorigenesis in xeroderma pigmentosum group a gene-deficient mice, Carcinogenesis 23 (2002) 627–633.
[42] H.J. Van der Fels-Klerx, L.C. Vermeulen, A.K. Gavai, C. Liu, Climate change impacts on aflatoxin B1 in maize and aflatoxin M1 in milk: a case study of maize grown in Eastern Europe and imported to the Netherlands, PLoS One 14 (2019) 6.
[43] S. El-Nahla, H. Imam, E. Moussa, A. Ibrahim, A. Ghanam, Teratogenic effects of aflatoxin in rabbits Oryctolagus Cuniculus, J. Vet. Anat. 6 (2) (2013) 67–85.
[44] V.C. Marina, B.T. Iamanaka, J.L. Pereira, D.P. Lemes, F. Nakano, M.H. Taniwaki,
Co-occurrence of ochratoxin a and aflatoxins in chocolate marketed in Brazil,
Food Control 26 (2012) 36–41.
[45] F.A. Ahmad, J.G. Gibbons, M.K. Lee, K.H. Han, S.B. Hong, Y.H. Yu, Controlling aflatoxin contamination and propagation of Aspergillus flavus by a soyfermenting Aspergillus oryzae strain, Sci. Rep. 8 (2018) 16871.
[46] M. Weidenborner, Encyclopedia of Food Mycotoxins, Springer Publisher Berlin,
New York, London, 2001.
[47] S. Ahlberg, D. Randolph, S. Okoth, J. Lindahl, Aflatoxin binders in foods for human consumption-can this be promoted safely and ethically? Toxins 11 (7) (2019) 410.
[48] V.C. Marina, B.T. Iamanaka, J.L. Pereira, D.P. Lemes, F. Nakano, M.H. Taniwaki,
Co-occurrence of ochratoxin a and aflatoxins in chocolate marketed in Brazil,
Food Control 26 (2012) 36–41.
[49] RASFF (The rapid alert system for food and feed) Annual report, European communities implications of mycotoxin in animal feed, Pak. J. Nat. 5 (2008)
398–403.
[50] L. Zengning, C. Jinfeng, Z. Xianghang, K. Weijun, Aflatoxin G1 reduces the molecular expression of HLA-I, TAP-1 and LMP-2 of adult esophageal epithelial cells in vitro, Toxicol. Lett. 195 (2010) 169–173.
[51] N. Yassein, Z.R. Zghair, Study of toxicity and pathogenicity of aflatoxin B1 and G1 in mice, Al-Anbar J. Vet. Sci. 5 (2012) 1999–6527.
[52] N. Yassein, Z.R. Zghair, Study of toxicity and pathogenicity of aflatoxin B1 and G1 in mice, Al-Anbar J. Vet. Sci. 5 (2012) 1999–6527.
[53] S. Peilu, G. Ningfei, W. Can, Z. Mei, Y. Li, L. Chunping, L. Kan, Aflatoxin G1 induced TNF-α-Dependent lung inflammation to enhance DNA damage in alveolar epithelial cells, J. Cell Physiol. 234 (2018) 9194–9206.
[54] M. Asi, S. Iqbal, A. Arino, ˜ A. Hussain, Effect of Seasonal variations and lactation times on aflatoxin M1 contamination in milk of different species from Punjab,
Pakistan, Food Control 25 (2012) 34–38.
[55] K.W. Lien, X. Wang, M.H. Pan, M.P. Ling, Assessing aflatoxin exposure risk from peanuts and peanut products imported to Taiwan, Toxins 11 (2019) 80.
[56] N.Y. Zhang, M. Qi, L. Zhao, M.K. Zhu, J. Guo, J. Liu, C.Q. Gu, S.A. Rajput, C.
S. Krumm, D.S. Qi, L.H. Sun, Curcumin prevents aflatoxin B1 hepatoxicity by inhibition of cytochrome P450 isozymes in Chick Liver, Toxins 8 (2016) 327.
[57] J. Vaid, R.K. Dawra, O.P. Sharma, S.S. Negi, Chronic aflatoxicosis in cattle, Vet.
Hum. Toxicol. 23 (1981) 436–438.
[58] Ramon, Gerardo, Aflatoxin Biochemistry and Molecular Biology Book, ISBN 978-
953-307-395-398.
[59] C. Afum, L. Cudjoe, J. Hills, R. Hunt, L. Padilla, S. Elmore, A. Afrije, et al.,
Association between aflatoxin M1 and liver disease in HBV/HCV infected persons in Ghana, Int. J. Environ. Res. Public Health 13 (4) (2016) 377.
[60] S. Marchese, A.A. Polo, S. Ariano, S. Velotto, S. Costantini, L. Severino, Aflatoxin
B.1 and M1: biological properties and their involvement in cancer development,
Toxins 10 (2018) 214.
[61] L. Xie, M. Li, D. Liu, X. Wang, P. Wang, H. Dai, Secalonic acid-F, a novel mycotoxin, represses the progression of hepatocellular carcinoma via MARCH 1 regulation of the PI3K/AKT/β-catenin signaling pathway, Molecules 24 (2019)
393.
[62] B. Tozzi, G. Liponi, V. Meucci, L. Casini, C. Dall’Asta, L. Intorre, D. Gatta,
Aflatoxins M1 and M2 in the milk of donkeys fed with naturally contaminated diet, Dairy Sci. Technol. 96 (2016) 513–523.
[63] D. Lee, L.G. Lee, Analysis of aflatoxin M1 and M2 in commercial dairy products using high-performance liquid chromatography with a fluorescence detector,
Food Control 50 (2015) 467–471.
[64] B. Barikbin, A. Allahresani, R. Khosravi, M. Khodadadi, Detection of aflatoxin M1 in dairy products marketed in Iran, J. Health Scope 4 (2015) 1.
[65] S.D. Saeger, A. Logrieco, in: Belgium, ToxinsReport from the 1st MYCOKEY
International Conference Global Mycotoxin Reduction in the Food and Feed
Chain Held in Ghent, 9, 2017, p. 276.
[66] Calvo, J. Cary, Association of fungal secondary metabolism and sclerotial biology,
Front. Microbiol. 6 (2015).
[67] P. Reddy, K. Guthridge, S. Vassiliadis, J. Hemsworth, I. Hettiarachchige,
G. Spangenberg, S. Rochfort, Tremorgenic mycotoxins: structure diversity and biological activity, Toxins 11 (2019) 302.
[68] L. Koz´ ak, Z. Szil´ agyi, L. Toth, ´ I. Pocsi, ´ I. Molnar, ´ Tremorgenic and neurotoxic paspaline-derived indole-diterpenes: biosynthetic diversity, threats and applications, Appl. Microbiol. Biotechnol. 103 (2019) 1599–1616.
[69] S. Zhang, B.J. Monahan, J.S. Tkacz, B. Scott, An indolediterpene gene cluster from
Aspergillus flavus, Appl. Environ. Microbiol. 70 (2004) 6875–6883.
[70] J.N. Matthew, B.K. Albert, J. Monahan, L.P. Beth, A. Gary, B. Scott, Identification of two aflatrem biosynthesis gene loci in Aspergillus flavus and metabolic engineering of Penicillium paxilli to elucidate their function, Appl. Environ.
Microbiol. 75 (2009) 7469–7481.
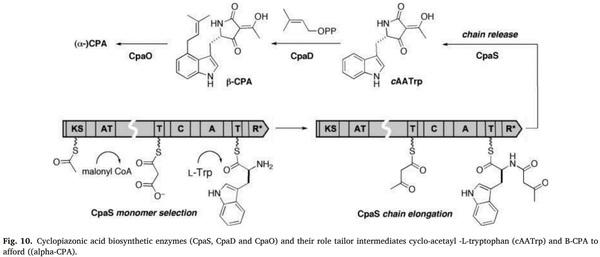
[71] K.J. Van der Merwe, P.S. Steyn, L. Fourie, Ochratoxin A., a toxic metabolite produced by Aspergillus ochraceusWilh, Nature 205 (1965) 1112–1113.
[72] W.E. Huff, P.B. Hamilton, Mycotoxins – their biosynthesis in fungi: ochratoxins – metabolites of combined pathways, J. Food Prot. 42 (1979) 815–820.
[73] L.L. Ana, Occurrence of ochratoxin a in coffee: threads and solutions-a minireview, Beverages 5 (2019) 36.
[74] W. Van Walbeek, P.M. Scott, J. Harwig, J.W. Lawrence, Penicillium viridicatum westling: a new source of Ochratoxin A, Can. J. Microbiol. 15 (1969) 1281–1285.
[75] Z. Liye, Z. Boyang, D. Yaqi, L. Hongyu, X. Wentao, A review: epigenetic mechanism in Ochratoxin A toxicity studies, Toxins 9 (2017) 113.
[76] K. Jørgensen, Survey of pork, poultry, coffee, beer and pulses for Ochratoxin A,
Food Addit. Contam. 15 (1998) 550–554.
[77] W. Dongmei, W. Xiaohu, X. Jun, D. Fenshou, Z. Yongquan, M. Ji, Determination of ochratoxin a contamination in grapes, processed grape products and animalderived products using ultra-performance liquid chromatography-tandem mass spectroscopy system, Sci. Rep. 8 (2018) 20–51.
[78] T.G. Ülger, A. Uçar, F.P. Çakıroglu, ˘ S. Yilmaz, Genotoxic effects of mycotoxins,
Toxicon 185 (2020) 104–113.
[79] A.D. Domijan, G. Gajski, I.N. Jovanovi, M. Geri, V. Garaj-Vrhovac, In vitro genotoxicity of mycotoxins ochratoxin A and fumonisin B1 could be prevented by sodium copper chlorophyllin–implication to their genotoxic mechanism, Food
Chem. 170 (2015) 455–462.
[80] A. Luhe, H. Hildebrand, U. Bach, T. Dingermann, H.J. Ahr, A new approach to studying ochratoxin A (OTA)-induced nephrotoxicity: expression profiling in vivo and in vitro employing cDNA microarrays, Toxicol. Sci. 73 (2) (2003) 315–328.
[81] J.G. Costa, N. Saraiva, P.S. Guerreiro, H. Louro, M.J. Silva, J.P. Miranda, et al.,
Ochratoxin A-induced cytotoxicity, genotoxicity and reactive oxygen species in kidney cells: an integrative approach of complementary endpoints, Food Chem.
Toxicol. 87 (2016) 65–76.
[82] R. Assunçao, M. Pinhao, S. Loureiro, P. Alvito, M.J. Silva, A multi-endpoint approach to the combined toxic effects of patulin and ochratoxin a in human intestinal cells, Toxicol. Lett. 313 (2019) 120–129.
[83] H.T. Marta, I.P. John, C.C. Marina, A.T. Aldir, T.I. Beatriz, Understanding mycotoxin contamination across the food chain in Brazil: challenges and opportunities, Toxins 11 (2019) 411.
[84] C. Jack, J. Lauren, J.L. Hyun, Z. Wei, A.T. Fadwa, Z. Jerry, D. Ryu, Occurrence of ochratoxin a in infant foods in the United States, J. Food Prot. 80 (2017)
251–256.
[85] A.A. Andrea, M.M.A. Pablo, P.F. Francisco, C. Gabriela, C. Cinthia, M.M. Kohil,
I. Peralta, et al., Occurrence of deoxynivalenol and Ochratoxin A in beers and wines commercialized in Paraguay, Toxins 11 (6) (2019) 308, 2019.
[86] L.N. Catootjie, A.H. Angi, M.A.J. Supit, S. Ambarwati, Aflatoxin and Ochratoxin A contamination in corn grains and sago putak meal from different sources for poultry in West Timor, Indonesia, Int. J. Poult. Sci. 18 (2019) 353–360.
[87] B. Terenzio, R. Marco, R. Silvia, G. Paola, Mycotoxins and related Fungi in Italian paddy rice during the growing season and storage, Toxins 11 (2019) 151.
[88] H. Shi, W. Schwab, P. Yu, Natural occurrence and co-contamination of twelve mycotoxins in industry-submitted cool-season cereal grains grown under a low heat unit climate condition, Toxins 11 (2019) 160.
[89] D.K. Halil, H. Canan, U. Beyza, Mycotoxin hazard in meat and meat products,
Atatürk Univ. J. Vet. Sci. 14 (1) (2019) 90–97.
[90] T. Cagla, K. Erhan, Determination of aflatoxin M1 and ochratoxin a in raw, pasteurized and UHT milk in Turkey, Acta Sci. Veterin 47 (2019) 1626.
[91] G. Jan, K. Robert, T. Magdalena, B.K. Anna, Occurrence and risk assessment of mycotoxins through polish beer consumption, Toxins 11 (2019) 254.
[92] F.A. Mohamed, G. Gozde, ¨ B. Terken, Mycotoxin detection in maize, commercial feed and raw dairy milk samples from Assiut City, Egypt. J. Vet. Sci. 6 (2019) 57.
[93] A. Juan-García, J. Tolosa, C. Juan, M.J. Ruiz, Cytotoxicity, genotoxicity,y and disturbance of cell cycle in HepG2 cells exposed to OTA and BEA: single and combined actions, Toxins 11 (2019) 341.
[94] R. Samson, S. Peterson, J. Frisvad, J. Varga, New species in Aspergillus section terrei, Stud. Mycol. 69 (2011) 39–55.
[95] M.B. Pildain, J.C. Frisvad, G. Vaamonde, D. Cabral, J. Varga, R.A. Samson, Two novel aflatoxin-producing Aspergillus species from Argentinean peanuts, Int. J.
Syst. Evol. Microbiol. 58 (2008) 725–735.
[96] C.P. Kurtzman, B.W. Horn, C.W. Hesseltine, Aspergillus nomius, a new aflatoxinproducing species related to Aspergillus flavus and Aspergillus tamarii, Antonie
Van Leeuwenhoek 53 (1987) 147–158.
[97] M.S. Madhyastha, R.V. Bhat, Aspergillus parasiticus growth and aflatoxin production on black and white pepper and the inhibitory action of their chemical constituents, Appl. Environ. Microbiol. 48 (1984) 376–379.
[98] A. Carvajal-Campos, A. Manizan, S. Tadrist, D. Akaki, R. Koffi-Nevry, G. Moore,
O.F. Stephen, et al., Aspergillus korhogoensis, a novel aflatoxin producing species from the Cote ˆ d’Ivoire, Toxins 9 (11) (2017) 353.
[99] J.C. Frisvad, P. Skouboe, R.A. Samson, Taxonomic comparison of three different groups of aflatoxin producers and a new efficient producer of aflatoxin B1, sterigmatocystin and 3-O-methylsterigmatocystin, Aspergillus rambellii sp. nov,
Syst. Appl. Microbiol. 28 (5) (2005) 442–453.
[100] J. Varga, J. Frisvad, R. Samson, A reappraisal of fungi producing aflatoxins, World
Mycotoxin J. 2 (2009) 263–277.
[101] G.G. Moore, R.A. Olarte, B.W. Horn, J.L. Elliott, R. Singh, C.J. Oneal, Global population structure and adaptive evolution of aflatoxin-producing Fungi, Ecol.
Evol. 7 (2017) 9179–9191.
[102] J. Varga, J. Frisvad, R. Samson, A reappraisal of fungi producing aflatoxins, World
Mycotoxin J. 2 (2009) 263–277.
[103] J.C. Frisvad, R.A. Samson, b. Emericella venezuelensis, a new species with stellate ascospores producing sterigmatocystin and Aflatoxin B1, Syst. Appl. Microbiol. 27 (2004) 672–680.
[104] T. Goto, D.T. Wicklow, Y. Ito, Aflatoxin and cyclopiazonic acid production by a sclerotium-producing Aspergillus tamarii strain, Appl. Environ. Microbiol. 62 (11) (1996) 4036–4038.
[105] H.P. Viaro, J.J.D. Silva, L.D.S. Ferranti, J.G. Bordini, F.P. Massi, M.H.P. Fungaro,
The first report of A. novoparasiticus, A. arachidicola and A. pseudocaelatus in
Brazilian Corn Kernels, Int. J. Food Microbiol. 243 (2017) 46–51.
[106] D. Domenico, M.P. Anna, P. Biancamaria, R. Alessandra, M. Oriana, Aspergillus affinis sp. nov., a novel ochratoxin A-producing Aspergillus species section circumdati isolated from decomposing leaves, Int. J. Syst. Evol. Microbiol. 62 (2012) 1007–1015.
[107] C.M. Visagie, J. Varga, J. Houbraken, M. Meijer, S. Kocsube, N. Yilmaz,
R. Fotedar, Ochratoxin production and taxonomy of the yellow aspergilli
Aspergillus section circumdati, Stud. Mycol. 78 (2014) 1–61.
[108] B. Paul, L.B. James, A.D. Mark, J.M. Themis, E.M. Noreen, Ochratoxin production by the Aspergillus ochraceus group and Aspergillus alliaceus, Appl. Environ.
Microbiol. 68 (2002) 2326–2329.
[109] C.W. Hesseltine, E.E. Vandegraft, D.I. Fennell, M.L. Smith, O.L. Shotwell,
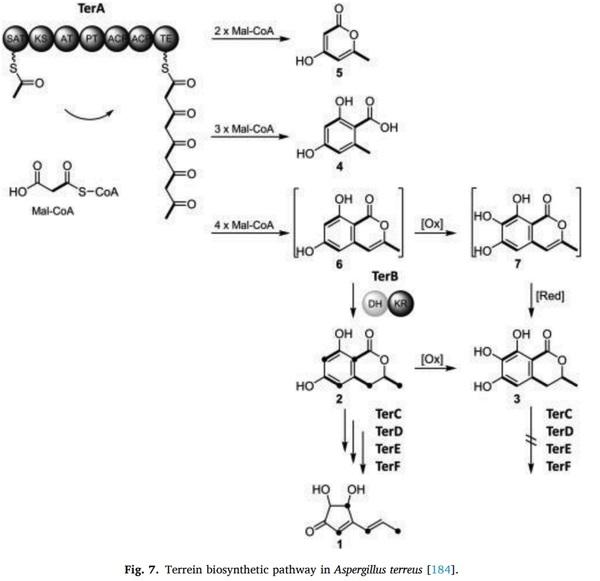
Aspergilli as ochratoxin producers, Mycologia 64 (1972) 539–550.
[110] A. Ciegler, Bioproduction of ochratoxin a and penicillc acid by members of the
Aspergillus ochraceus group, Can. J. Infect. Dis. Med. Microbiol. 18 (1972)
631–636.
[111] G. Perrone, A. Susca, G. Cozzi, K. Ehrlich, J. Varga, J.C. Frisvad, Biodiversity of
Aspergillus species in some important agricultural products, Stud. Mycol. 59 (2007) 53–66.
[112] R. Samson, J. Houbraken, A. Kuijpers, J. Frank, J. Frisvad, New ochratoxin a or sclerotium producing species in Aspergillus section nigri, Stud. Mycol. 50 (2004)
45–61.
[113] M. Angel, ´ R. Mateo, F.M. Valle-Algarra, E.M. Mateo, M. Jim´enez, Effect of
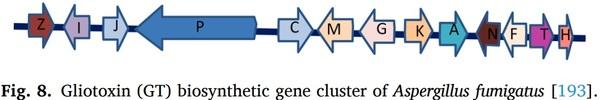
Carbendazim and physicochemical factors on the growth and ochratoxin a production of aspergilluscarbonarius isolated from grapes, Int. J. Food Microbiol.
119 (3) (2007) 230–235.
[114] J.D. Palumbo, T.L. O’Keeffe a, N.E. Mahoney, Inhibition of ochratoxin a production and growth of Aspergillus species by phenolic antioxidant compounds,
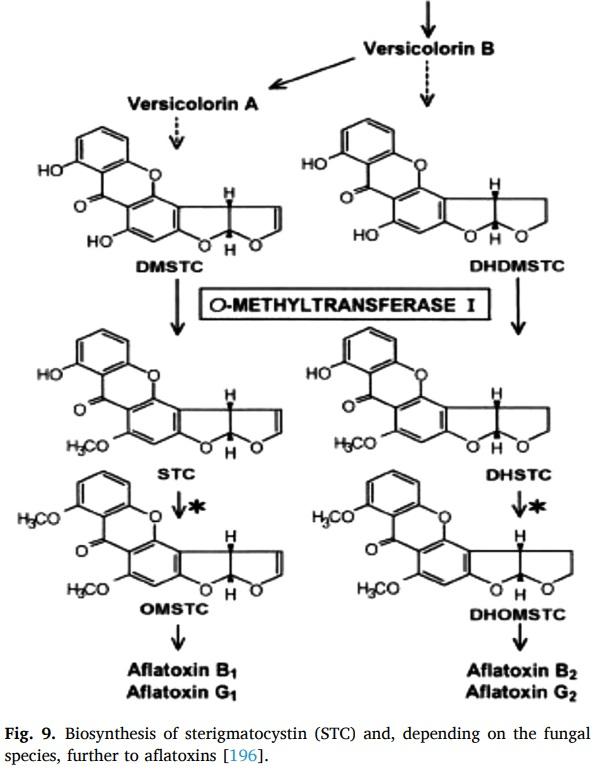
Mycopathologia 164 (2007) 241–248.
[115] J.C. Frisvad, M. Frank, J.A.M.P. Houbraken, A.F.A. Kuijpers, R.A. Samson, a. New ochratoxin A producing species of Aspergillus section circumdati, Stud. Mycol. 50 (2004) 23–43.
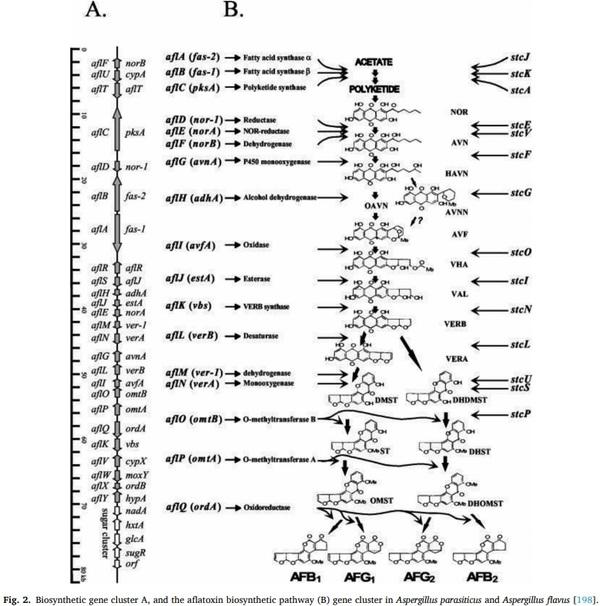
[116] J. Frisvad, V. Hubka, C. Ezekiel, S.B. Hong, A. Novakov ´ a, ´ A. Chen, M. Arzanlou, et al., Taxonomy of Aspergillus section flavi and their production of aflatoxins, ochratoxins, and other mycotoxins, Stud. Mycol. 93 (2019) 1–63.
[117] M. Arzanlou, R. Samadi, J.C. Frisvad, J. Houbraken, Y. Ghosta, Two novel
Aspergillus Species from hypersaline soils of the national park of Lake Urmia,
Iran, Mycol. Prog. 15 (2016) 1081–1092.
[118] Y.N. Li, Y.Y. Wang, Y.Q. Zheng, Y.H. Guo, Preparation and characterization of the high specificity monoclonal antibodies against citrinin, Prog. Biochem. Biophys.
37 (2011) 1248–1253.
[119] M.P. Artigot, N. Loiseau, J. Laffitte, L. Mas-Reguieg, S. Tadrist, I.P. Oswald,
O. Puel, Molecular cloning and functional characterization of two CYP619 cytochrome P450s involved in biosynthesis of patulin in Aspergillus clavatus,
Microbiology 155 (5) (2009) 1738–1747.
[120] J. Varga, M. Due, J. Frisvad, R. Samson, Taxonomic revision of Aspergillus section clavati based on molecular, morphological and physiological data, Stud. Mycol.
59 (2007) 89–106.
[121] R. Steiman, F. Seigle-Murandi, L. Sage, S. Krivobok, Production of patulin by micromycetes, Mycopathology 105 (1989) 129–133.
[122] H.K. Frank, Occurrence of Patulin in fruit and vegetables, Annales de la Nutrition et de L’Alimentation 31 (1977) 459–465.
[123] M.I. Luque, A. Rodríguez, M.J. Andrade, R. Gordillo, M. Rodríguez, J.J. Cordoba, ´
Development of a PCR protocol to detect patulin producing moulds in food products, Food Control 22 (2011) 1831–1838.
[124] J. Frisvad, A critical review of producers of small lactone mycotoxins: patulin, penicillic acid, and moniliformin, World Mycotoxin J. 11 (2018) 73–100.
[125] P.K. Chang, K. Ehrlich, I. Fujii, Cyclopiazonic acid biosynthesis of Aspergillus flavus and Aspergillus oryzae, Toxins 1 (2) (2009) 74–99.
[126] C. Rank, M.L. Klejnstrup, L.M. Petersen, S. Kildgaard, J.C. Frisvad, C.
H. Gotfredsen, O.L. Thomas, Comparative chemistry of Aspergillus oryzae RIB40 and A. flavus NRRL 3357, Metabolites 2 (2012) 39–56.
[127] K. Sun, Y. Li, L. Guo, Y. Wang, P. Liu, W. Zhu, Indole diterpenoids and
Isocoumarin from the fungus, Aspergillus flavus, isolated from the prawn, Penaeus vannamei, Mar. Drugs 12 (2014) 3970–3981.
[128] V. Uka, G. Moore, N. Arroyo-Manzanares, D. Nebija, S.D. Saeger, J.D.D. Mavungu,
Unravelling the diversity of the cyclopiazonic acid family of mycotoxins in
Aspergillus flavus by UHPLC Triple-TOF HRMS, Toxins 9 (2017) 35.
[129] Y. Ito, S.W. Peterson, D.T. Wicklow, T. Goto, Aspergillus pseudotamarii, a new aflatoxin producing species in Aspergillus section flavi, Mycol. Res. 105 (2001)
233–239.
[130] R. Krska, K. ahrer, ¨ L. Richard, I. Rodrigues, R. Schuhmacher, B. Slate, W.
B. Whitaker, in: E.M. Binder, R. Krasha (Eds.), Guide to Mycotoxins Featuring
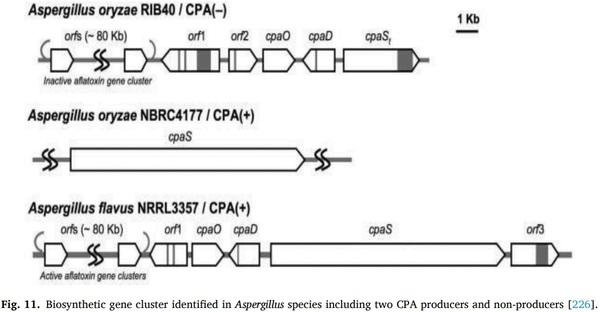
Mycotoxin Risk Management in Animal Production, biomin edition, Romar labs
Division holding GmbH, Australia, 2012. ISBN 9780-9573721-1-5.
[131] T.O. Larsen, J. Smedsgaard, K.F. Nielsen, M.A.E. Hansen, R.A. Samson, J.
C. Frisvad, Production of mycotoxins by Aspergillus lentulus and other medically important and closely related species in section fumigati, Med. Mycol. 45 (2007)
225–232.
[132] R. Orth, Mycotoxins of Aspergillus oryzae strains for use in the food industry as starters and enzyme producing molds, Annales de la Nutrition et de
L’alimentation 31 (1977) 617–624.
[133] R.J. Cole, Cyclopiazonic acid and related toxins, in: V. Betina (Ed.), MycotoxinsProduction, Isolation, Separation, and Purification, Elsevier Science Publishers,
Amsterdam, the Netherlands, 1984, pp. 405–414.
[134] N.G. Vinokurova, N.E. Ivanushkina, I.I. Khmel’Nitskaya, M.U. Arinbasarov,
Synthesis of α-cyclopiazonic acid by fungi of the genus Aspergillus, Appl. Biochem.
Microbiol. 43 (2007) 435–438.
[135] M. Yamazaki, Y. Maebayashi, K. Miyaki, The isolation of secalonic acid a from
Aspergillus ochraceus cultured on rice, Chem. Pharm. Bull. 19 (1971) 199–201.
[136] I. Kurobane, L.C. Vining, A.G. McInnes, A new secalonic acid linkage between tetrahydroxan- thone units determined from deuterium isotope 13C chemical shifts, Tetrahedron Lett. (1978) 4633–4636.
[137] I. Yosioka, H. Yamauchi, K. Murata, I. Kitagawa, Coloring substances of a lichen cetrariaornata, Chem. Pharm. Bull. 20 (1972) 1082–1084.
[138] I. Yosioka, T. Nakanishi, S. Izumi, I. Kitagawa, Structure of a lichen pigment entothein and its identity with secalonic acid a, a major ergot pigment, Chem.
Pharm. Bull. 16 (1968) 2090–2091.
[139] H. Ren, L. Tian, Q. Gu, W. Zhu, Secalonic acid d; a cytotoxic constituent from marine lichen-derived fungus gliocladium sp. T31, Arch. Pharm. Res. 29 (2006)
59–63.
[140] R. Samson, P. Noonim, M. Meijer, J. Houbraken, J. Frisvad, J. Varga, Diagnostic tools to identify black aspergilli, Stud. Mycol. 59 (2007) 129–145.
[141] R. Andersen, G. Buechi, B. Kobbe, A.L. Demain, Secalonic acids d and F are toxic metabolites of Aspergillus aculeatus, J. Org. Chem. 42 (2) (1977) 352–353.
[142] P. Steyn, The Isolation, Structure and absolute configuration of secalonic acid d, the toxic metabolite of Penicillium oxalicum, Tetrahedron 26 (1970) 51–57.
[143] L. Du, Q. Zhang, L. Chen, Y. Bi, Y.P. Li, X.X. Li, L. Qin-Yan, et al., Secalonic acids
H and I, two new secondary metabolites from the marine-derived fungus
Penicillium oxalicum, Heterocycles 94 (9) (2017) 17–66.
[144] M.R. Tepaske, J.B. Gloer, D.T. Wicklow, P.F. Dowd, Aflavarin and β-Aflatrem: new anti-insectan metabolites from the sclerotia of Aspergillus flavus, J. Nat. Prod.
55 (1992) 1080–1086.
[145] W.J. Lan, K.T. Wang, M.Y. Xu, J.J. Zhang, C.K. Lam, G.H. Zhong, De-Po Yang, et al., Secondary metabolites with chemical diversity from the marine-derived fungus pseudallescheria boydii F19-1 and their cytotoxic activity, R. Soc. Chem.
Adv. 6 (2016) 76206–76213.
[146] W.B. Yin, A. Grundmann, J. Cheng, S.M. Li, Acetylaszonalenin biosynthesis in
Neosartorya fischeri. Identification of the biosynthetic gene cluster by genomic mining and functional proof of the genes by biochemical investigation, J. Biol.
Chem. 284 (2009) 100–109.
[147] T. Boruta, M. Bizukojc, Culture-based and sequence-based insights into biosynthesis of secondary metabolites by Aspergillus terreus ATCC 20542,
J. Biotechnol. 175 (2014) 53–62.
[148] I.H. Qureshi, A. Kamal, R. Noorani, S.A. Husain, Studies in the biochemistry of microorganisms, Part vii. Terrein and kojic acid, metabolic products of Aspergillus stellatusCurzi, Pak. J. Biol. Sci. 4 (1968) 367.
[149] C.J. Rabie, M. Steyn, C.G. van Schalkwyk, New species of Aspergillus producing sterigmatocystin, Appl. Environ. Microbiol. 33 (1997) 1023–1025.
[150] Z. ˇ Jurjevi´c, S.W. Peterson, M. Solfrizzo, M. Peraica, Sterigmatocystin production by nine newly described Aspergillus species in section versicolores grown on two different media, Mycotoxicol. Res. 29 (2013).
[151] C.C. Tsang, T.W.S. Hui, K.C. Lee, J.H.K. Chen, A.H.Y. Ngan, E.W.T. Tam, J.F.
W. Chan, Genetic diversity of Aspergillus species isolated from Onychomycosis and aspergillushongkongensis sp. Nov. with implications to antifungal susceptibility testing, Diagn. Microbiol. Infect. Dis. 84 (2016) 125–134.
[152] D.D. Jakˇsi´c, S. Kocsub´e, O. Bencsik, A. Kecskem´eti, A.A. Szekeres, C. V´ agvolgyi, ¨
J. Varga, Species diversity and cytotoxic potency of airborne sterigmatocystinproducing Aspergilli from the section versicolores, Sci. Total Environ. 562 (2016)
296–304.
[153] H.W. Schroeder, W.H. Kelton, Production of Sterigmatocystin by some species of the genus Aspergillus and its toxicity to chicken embryos, Appl. Environ.
Microbiol. 30 (1975) 589–591.
[154] V. Hubka, A. Novakov ´ ´ a, A.S.W. Peterson, A reappraisal of Aspergillus section nidulantes with descriptions of two new sterigmatocystin-producing species,
Plant Syst. Evol. 302 (2016) 1267–1299.
[155] J.C. Frisvad, Secondary Metabolites as an Aid to Emericella Classification.
Advances in Penicillium and Aspergillus Systematics, Vol. 102, Plenum Press,
New York, 1985, pp. 437–443.
[156] G.A. Glister, T.I. Williams, Production of Gliotoxin by Aspergillus fumigatus mut.
Helvola Yuill, Nature 153 (1944), 651–651.
[157] E.P. Reeves, C. Messina, S. Doyle, K. Kavanagh, Correlation between gliotoxin production and virulence of Aspergillus fumigatus in Galleria mellonella,
Mycopathology 158 (2004) 73–79.
[158] S. Wilkinson, J.F. Spilsbury, Gliotoxin from Aspergillus chevalierimangin thom et church, Nature 206 (1965), 619–619.
[159] T.R. Bui-Klimke, F. Wu, Ochratoxin a and human health risk: a review of the evidence, Crit. Rev. Food Sci. Nutr. 55 (2015) 1860–1869.
[160] J.C. Gautier, J. Richoz, D.H. Welti, Metabolism of ochratoxin a: absence of formation of genotoxic derivatives by human and rat enzymes, Chem. Res.
Toxicol. 14 (1) (2001) 34–45.
[161] J.E. Jennings-Gee, M. Tozlovanu, R. Manderville, M.S. Miller, A. PfohlLeszkowicz, G.G. Schwartz, Ochratoxin a: in utero exposure in mice induces adducts in testicular DNA, Toxins Basel 2 (2010) 1428–1444.
[162] K. Stemmer, H. Ellinger-Ziegelbauer, H.J. Ahr, D.R. Dietrich, Molecular characterization of preneoplastic lesions provides insight on the development of renal tumors, Am. J. Pathol. 175 (2009) 1686–1698.
[163] A.C. Hetherington, H. Raistrick, On the Production and Chemical Constitution of a New Yellow Colouring Matter, Citrinin, Produced from Glucose by Penicillium citrinum Thom. Philosop, Royal Society B: Biological Sciences 220 (1931)
269–295.
[164] L.M.A. Barnett, B.S. Cummings, Nephrotoxicity and renal pathophysiology: a contemporary perspective, Toxicology Science 164 (2018) 379–390.
[165] J.H. Doughari, The occurrence, properties and significance of citrinin mycotoxin,
J. Plant Pathol. Microbiol. 6 (2015) 1–6.
[166] L. Gayathri, R. Dhivya, D. Dhanasekaran, V.S. Periasamy, A.A. Alshatw, M.
A. Akbarsha, Hepatotoxic effect of ochratoxin a and Citrinin, alone and in combination, and protective effect of vitamin e: in vitro study in HepG2 cell, Food
Chem. Toxicol. 83 (2015) 151–163.
[167] L.M.A. Barnett, B.S. Cummings, Nephrotoxicity and renal pathophysiology: a contemporary perspective, Toxicology Science 164 (2018) 379–390.
[168] V. Ostry, F. Malir, J. Ruprich, Producers and important dietary sources of ochratoxin a and citrinin, Toxins 5 (2013) 1574–1586.
[169] B. Culig, M. Berardi, J. Bosnir, S. Serdar, D. Lasic, A. Racs, A. Galic, et al.,
Presence of Citrinin in grains and its possible health effects, Afr. J. Tradit.
Complement. Altern. Med. 14 (2017) 22–30.
[170] P.J. Blanc, J.P. Laussac, J. Le Bars, P. Le Bars, M.O. Loret, A. Pareilleux, D. Prome, et al., Characterization of monascidin a from Monascus as citrinin, Int. J. Food
Microbiol. 27 (1995) 201–213.
[171] R.A. Hill, R.H. Carter, J. Staunton, Biosynthesis of Fungal Metabolites. Terrein, A
Metabolite ofAspergillus terreus Thom, J. Chem. Soc. Perkin Trans. I 1 (1981)
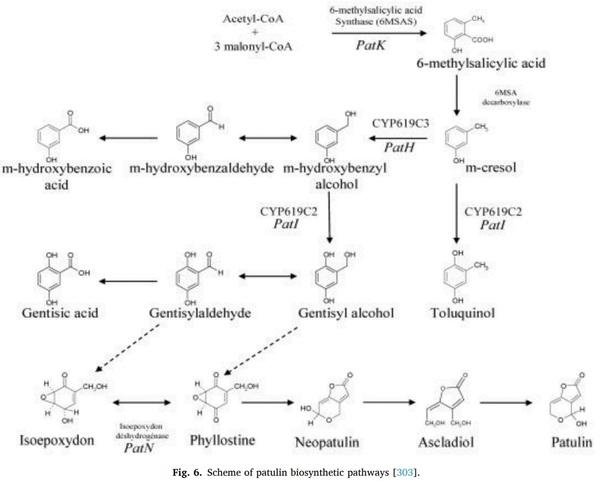
2570–2576.
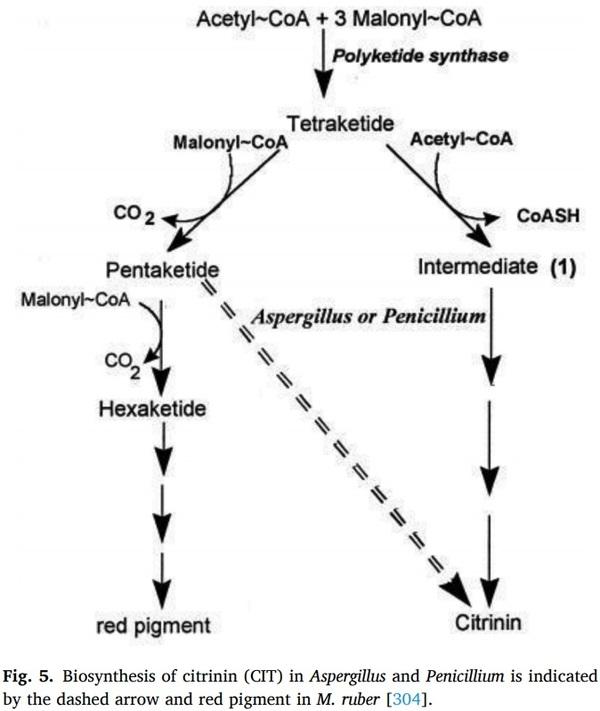
[172] A. Erdogan, ˘ D. Ghimire, M. Gürses, B. Çetin, A. Baran, Patulin contamination in fruit juices and its control measures, European Journal of Science and Technology
14 (2018) 39–48.
[173] I. Chalmers, Commentary: the 1944 patulin trial: the first properly controlled multicentre trial conducted under the aegis of the british medical research council, Int. J. Epidemiol. 33 (2004) 253–260.
[174] M. Kim, S. Shukla, Y. Oh, S.H. Chung, M. Kim, Comparative diminution of patulin content in apple juice with food-grade additives sodium bicarbonate, vinegar, mixture of sodium bicarbonate and vinegar, citric acid, baking powder, and ultraviolet irradiation, Front. Pharmacol. 9 (2018) 822.
[175] A. Guerra-Moreno, J. Hanna, Induction of proteotoxic stress by the mycotoxin patulin, Toxicol. Lett. 276 (2017) 85–91.
[176] G. Speijers, M. Franken, F.V. Leeuwen, Sub-acute toxicity study of Patulin in the rat: effects on the kidney and the gastro-intestinal tract, Food Chem. Toxicol. 26 (1988) 23–30.
[177] B. Zhang, X. Peng, G. Li, Y. Xu, X. Xia, Q. Wang, Oxidative stress is involved in patulin induced apoptosis in HEK293 cells, Toxicon 94 (2015) 1–7.
[178] S. Pal, N. Singh, K.M. Ansari, Toxicological effects of patulin mycotoxin on the mammalian system: an overview, Toxicol. Res. 6 (2017) 764–771.
[179] E. Song, X. Xia, C. Su, W. Dong, Y. Xian, W. Wang, S. Yong, Hepatotoxicity and genotoxicity of Patulin in mice, and its modulation by green tea polyphenols administration, Food Chem. Toxicol. 71 (2014) 122–127.
[180] H. Malekinejad, J. Aghazadeh-Attari, A. Rezabakhsh, M. Sattari,
B. Ghasemsoltani-Momtaz, Neurotoxicity of mycotoxins produced in vitro by
Penicillium roqueforti isolated from maize and grass silage, Hum. Exp. Toxicol. 34 (2015) 997–1005.
[181] M. Boussabbeh, A. Prola, I.B. Salem, A. Guilbert, H. Bacha, C. Lemaire, S. AbisEssefi, Crocin and quercetin prevent PAT-Induced apoptosis in mammalian cells: involvement of ROS-Mediated ER stress pathway, Environ. Toxicol. 31 (2015)
1851–1858.
[182] I. Ayed-Boussema, J.M. Pascussi, P. Maurel, H. Bacha, W. Hassen, Effect of aflatoxin B1 on nuclear receptors PXR, CAR, and AhR and their target cytochromes P450 mRNA expression in primary cultures of human hepatocytes,
Int. J. Toxicol. 31 (2012) 86–93.
[183] F. Lynen, M. Tada, Die BiochemischenGrundlagen der polyacetat-regel, Angew.
Chem. 73 (1961) 513–519.
[184] C. Zaehle, M. Gressler, E. Shelest, E. Geib, C. Hertweck, M. Brock, Terrein biosynthesis in Aspergillus terreus and its impact on phytotoxicity, Chem. Biol. 21 (2014) 719–731.
[185] M. Arakawa, T. Someno, M. Kawada, D. Lkeda, A new terrein glucoside, a novel inhibitor of angiogenin secretion in tumor angiogenesis, J. Antibiot. 61 (2007) (2008) 442–448.
[186] M. Demasi, A.L. Felicio, A.O. Pacheco, H. Leite, C. Lima, L.H. Andrade, Studies on terrein as a new class of proteasome inhibitors, J. Braz. Chem. Soc. 21 (2010)
299–305.
[187] W.Y. Liao, C.N. Shen, L.H. Lin, Y.L. Yang, H. Hsin-Ying, C. Jing-Wei, ShengChu Kuo, Asperjinone, a nor-neolignan, and terrein, a suppressor of ABCG2- expressing breast cancer cells, from thermophilic Aspergillus terreus, J. Nat. Prod.
75 (2012) 630–635.
[188] M. Hiroki, O. Kazuhiro, Y. Daisuke, T. Toki, M. Kyouta, Y. Satoshi, M. Koichi, et al., Synthetic + - terrein suppresses Interleukin-6/Soluble Interleukin-6 receptor induced-secretion of vascular endothelial growth factor in human gingival fibroblasts, Bioorg. Med. Chem. 22 (2014) 5338–5344.
[189] R. Weindling, O.H. Emerson, The isolation of a toxic substance from the culture filtrate of Trichoderma, Phytopathology 26 (1936) 1068.
[190] R. Weindling, Experimental consideration of the mold toxins of Gliocladium and
Trichoderma, Phytopathology 31 (1941) 991.
[191] A. Müllbacher, P. Waring, U. Tiwari-Palni, R.D. Eichner, Structural relationship of
Epipolythiodioxopiperazines and their immunomodulating activity, Mol.
Immunol. 23 (1986) 231–235.
[192] D.M. Gardiner, A.J. Cozijnsen, L.M. Wilson, M.S.C. Pedras, B.J. Howlett, The sirodesmin biosynthetic gene cluster of the plant pathogenic fungus leptosphaeriamaculans, Mol. Microbiol. 53 (2004) 1307–1318.
[193] D.M. Gardiner, B.J. Howlett, Bioinformatics and expression analysis of the putative gliotoxin biosynthetic gene cluster of Aspergillus fumigatus, FEMS
Microbiol. Lett. 248 (2005) 241–248.
[194] M.A. Nouri, M.M. Al-Halbosiy, B.I. Dheeb, A.J. Hashim, Cytotoxicity, and genotoxicity of Gliotoxin on human lymphocytes in vitro, J. King Saud Univ. Sci.
27 (2015) 193–197.
[195] A. VersiIlovskis, S.D. Saeger, Sterigmatocystin occurrence in foodstuffs and analytical methods an overview, Mol. Nutr. Food Res. 54 (2010) 136–147.
[196] A.S. Rofiat, F. Fanelli, O. Atanda, M. Sulyok, G. Cozzi, S. Bavaro, R. Krska,
A. Logrieco, C.N. Ezekiel, Fungal and bacterial metabolites associated with natural contamination of locally processed rice in Nigeria, Food Addit. and
Contam. 32 (2015) 950–959.
[197] N. Kobayashi, A. Kubosaki, Y. Takahashi, M. Yanai, R. Konuma, S. Uehara,
Distribution of Sterigmatocystin-Producing Aspergilli in Japan, Food Saf. 6 (2018) 67–73.
[198] J. Yu, P.K. Chang, K.C. Ehrlich, J.W. Cary, D. Bhatnagar, T.E. Cleveland, G.
A. Payne, Clustered pathway genes in aflatoxin biosynthesis, Appl. Environ.
Microbiol. 703 (2004) 1253–1262.
[200] K. Noda, M. Umeda, Y. Ueno, Cytotoxic and mutagenic effects of Sterigmatocystin on cultured chinese Hamster cells, Carcinogenesis 2 (1981) 945–949.
[201] P.T. Curry, R.N. Reed, R.M. Martino, R.M. Kitchin, Induction of sister-chromatid exchanges in vivo in mice by the mycotoxins Sterigmatocystin and griseofulvin,
Mutat. Res. Genet. Toxicol. Environ. Mutagen. 137 (1984) 111–115.
[202] N. Ueda, K. Fujie, K. Gotoh-Mimura, S. Chattopadhyay, T. Sugiyama, Acute cytogenetic effect of Sterigmatocystin on rat bone-marrow cells in vivo, Mutat.
Res. Lett. 139 (1984) 203–206.
[203] N. Zouaoui, B. Mallebrera, H. Berrada, S. Abid-Essefi, H. Bacha, M. Ruiz,
Cytotoxic effects induced by Patulin, Sterigmatocystin, and Beauvericin on
CHO–K1 cells, Food Chem. Toxicol. 89 (2016) 92–103.
[204] S. Huang, J. Wang, L. Xing, H. Shen, X. Yan, J. Wang, X. Zhang, Impairment of cell cycle progression by Sterigmatocystin in human pulmonary cells in vitro, Food
Chem. Toxicol. 66 (2014) 89–95.
[205] X. Jiang, J. Wang, L. Xing, H. Shen, W. Lian, L. Yi, D. Zhan, et al.,
Sterigmatocystin-induced checkpoint adaptation depends on Chk1 in immortalized human gastric epithelial cells in vitro, Arch. Toxicol. Suppl. 91 (2016) 259–270.
[206] K. Balogh, B. Kovesi, ¨ E. Zandoki, ´ S. Kulcs´ ar, Z. Ancsin, M. Erd´elyi, C. Dobolyi, et al., Effect of sterigmatocystin or aflatoxin contaminated feed on lipid peroxidation and glutathione redox system and expression of glutathione redox system regulatory genes in broiler chicken, Antioxidants 8 (7) (2019) 201.
[207] V. Gao, L. Jiang, L. Ge, M. Chen, C. Geng, G. Yang, L. Qiujuan, et al.,
Sterigmatocystin-induced oxidative DNA damage in human liver-derived cell line through lysosomal damage, Toxicology 29 (2015) 1–7.
[208] V. Sivakumar, J. Thanislass, S. Niranjali, H. Devaraj, Lipid peroxidation as a possible secondary mechanism of sterigmatocystin toxicity, Hum. Exp. Toxicol.
20 (2001) 398–403.
[209] EFSA (European Food Safety Authority), Scientific opinion on the risk for public and animal health related to the presence of Sterigmatocystin in food and feed,
Eur. Food Saf. Authority J. 11 (2013) 32–54.
[210] V. Ostry, J. Toman, Y. Grosse, F. Malir, Cyclopiazonic acid: 50th anniversary of its discovery, World Mycotoxin J. 11 (2018) 135–148.
[211] S. Okoth, M.D. Boevre, A. Vidal, J.D.D. Mavungu, S. Landschoot, M. Kyallo,
J. Njuguna, J. Harvey, S. De Saeger, Genetic and toxigenic variability within
Aspergillus flavus population isolated from maize in two diverse environments in
Kenya, Front. Microbiol. 9 (2018) 57.
[212] C.M. Maragos, K.K. Sieve, J. Bobell, Detection of cyclopiazonic acid CPA in maize by immunoassay, Mycotoxin Res. 33 (2017) 157–165.
[213] D. Heperkan, S. Somuncuoglu, F. Karbancioglu-Güler, N. Mecik, Natural contamination of cyclopiazonic acid in dried figs and co-occurrence of aflatoxin,
Food Control 23 (2012) 82–86.
[214] B.L. Rao, A. Husain, Presence of cyclopiazonic acid in kodo millet paspalumscrobiculatum causing kodua poisoning in man and its production by associated Fungi, Mycopathology 89 (1985) 177–180.
[215] K. Hariprasanna, Genetic improvement in kodo millet, in: V.A. Tonapi, J.V. Patil (Eds.), Book Millets: Ensuring Climate Resilience and Nutritional Security, Daya
Publishing House, New Delhi, 2015.
[216] U. Rasheed, H. Wu, J. Wei, X. Ou, P. Qin, X. Yao, H. Chen, A polyphasic study of
Aspergillus section flavi isolated from corn in Guangxi, China- a hot spot of aflatoxin contamination, Int. J. Food Microbiol. 310 (2019) 108–307.
[217] T.L. Goron, M.N. Raizada, Genetic diversity and genomic resources available for the small millet crops to accelerate a new green revolution, Front. Plant Sci. 6 (2015).
[218] S. Dwivedi, H. Upadhyaya, S. Senthilvel, C. Hash, K. Fukunaga, X. Diao, D. Santra, et al., Millets RASFF: genetic and genomic resources, Plant Breed. Rev. (2011)
247–375.
[219] A. Gallo, G. Giuberti, J. Frisvad, T. Bertuzzi, K. Nielsen, Review on mycotoxin issues in ruminants: occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects, Toxins 7 (8) (2005) 3057–3111.
[220] M.M. Deabes, Natural Co-occurrence of aflatoxins, cyclopiazonic acid, and their production by Aspergillus flavus isolates from corn grown in Egypt, Adv. Clin.
Toxicol. 3 (2018) 3.
[221] C. Maragos, Complexation of the mycotoxin cyclopiazonic acid with lanthanides yields luminescent products, Toxins 10 (2018) 285.
[222] S. Sugiharto, A review of filamentous fungi in broiler production, Ann. Agric. Sci.
64 (2019) 1–8.
[223] E.D. King, A.B. Bassi, D.C. Ross, B. Druebbisch, An industry perspective on the use of “Atoxigenic” strains of Aspergillus flavus as biological control agents and the significance of cyclopiazonic acid, Toxin Rev. 30 (2011) 33–41.
[224] J. Dupont, S. Dequin, T. Giraud, F. Tacon, S. Marsit, J. Ropars, F. Richard, et al.,
Fungi as a source of food, Microbiol. Spectrum 5 (2) (2017) 0030–2016.
[225] C.W. Holzapfel, D.C. Wilkins, On the biosynthesis of cyclopiazonic acid,
Phytochemistry 10 (1971) 351–358.
[226] P.K. Chang, K.C. Ehrlich, S.S. Hua, Cladal relatedness among Aspergillus oryzae isolates and Aspergillus flavus S and l morphotype isolates, Int. J. Food Microbiol.
108 (2006) 172–177.
[227] W. Zhang, K. Krohn, Z. Ullah, U. Florke, ¨ G. Pescitelli, L. Di Bari, A. Sandor, New mono- and dimeric members of the secalonic acid family: blennolides a–g isolated from the fungus blennoria sp, Chem. Eur. J. 14 (2008) 4913–4923.
[228] T. Wezeman, S. Br¨ ase, K.S. Masters, Xanthone Dimers: A Compound Family which is both Common and Privileged, Nat. Prod. Rep. 32 (2015) 1–104.
[229] B. Franck, H. Flasch, Die ErgochromePhysiologie, Isolierung, Struktur und
Biosynthese, Progress in the Chem.of Org. Nat. Prodt., 1973, pp. 151–206.
[230] A. Zhai, X. Zhu, X. Wang, R. Chen, H. Wang, Secalonic acid a protects dopaminergic neurons from 1-methyl-4-phenylpyridinium MPP + -Induced cell death via the mitochondrial apoptotic pathway, Eur. J. Pharmacol. 71 (2013)
58–67.
[231] K.S. Masters, S. Brase, ¨ Xanthones from Fungi, lichens, and Bacteria: the natural products and their synthesis, Chem. Rev. 112 (2012) 3717–3776.
[232] T. Qin, J.A. Porco, Total syntheses of secalonic acids a and d, AngewandteChemie
126 (2014) 3171–3174.
[233] M. Barbero, E. Artuso, C. Prandi, Fungal anticancer metabolites: synthesis towards drug discovery, Curr. Med. Chem. 25 (2017).
[234] S. Kanematsu, A.S. Franz, M.S. Miyono, Antitumor activity of cytoxan, Cancer
Res. 21 (1961) 1412–1420.
[235] M. Flieger, E. Stodůlkov´ a, S.A. Wyka, J. Cerný, ˇ V. Grobarov ´ a, ´ K. Píchov´ a,
P. Novak, et al., Ergochromes: heretofore neglected side of ergot toxicity, Toxins
11 (8) (2019) 439.
[236] D. Ganapathy, J.R. Reiner, L.E. Loeffler, L. Ma, B. Gnanaprakasam, B. Niepoetter,
I. Koehne, et al., ChemInform abstract: enantioselective total synthesis of secalonic acid E, Cheminform 47 (2015) 15.
[237] S.K. Guru, A.S. Pathania, S. Kumar, D. Ramesh, M. Kumar, S. Rana, A. Kumar, et al., Secalonic Acid-D represses HIF1/VEGF-Mediated angiogenesis by regulating the Akt/mTOR/p70S6K signaling cascade, Cancer Res. 75 (14) (2015)
2886–2896.
[238] Z. Guo, Z. She, C. Shao, L. Wen, F. Liu, Z. Zheng, et al., 1H and 13C NMR signal assignments of paecilin A and B, two new chromone derivatives from mangrove endophytic fungus Paecilomycessp. Tree 1–7, Magn. Reson. Chem. 45 (9) (2007)
777–780.
[239] H.J. Cho, M.J. Jung, S. Woo, J. Kim, E.S. Lee, Y. Kwon, N. Younghwa, New benzoxanthone derivatives as topoisomerase inhibitors and DNA cross-linkers,
Bioorg. Med. Chem. 18 (3) (2010) 1010–1017.
[240] L. Chen, Y.P. Li, X. Li, Z.H. Lu, Q.H. Zheng, Q.Y. Liu, Isolation of 4,4′ -Bond secalonic acid d from the marine-derived fungus Penicillium oxalicum with inhibitory property against hepatocellular carcinoma, J. Antibiot. 72 (1) (2018)
34–44.
[241] R.P. Farhana, J.B. David, M.P. Amy, K. Konstantinos, S. Sanjay, J.S. Horst,
W. David, Systems biology and multi-omics integration: viewpoints from the metabolomics research community, Metabolites 9 (2019) 76.
[242] M. Castro-Puyana, R. P´erez-Míguez, L. Montero, M. Herrero, Application of mass spectrometry-based metabolomics approaches for food safety, quality, and traceability, TrAC Trends Anal. Chem. 93 (2017) 102–118.
[243] L. Righetti, C. Dall’Asta, J. Hajslova, J. Rubert, Metabolomics Approaches and
Their Hidden Potential for Explaining the Mycotoxin Contamination Problem,
Metabolomics-fundamentals and Applications, IntechOpen, 2016.
[244] P.M. Scott, J.W. Lawrence, W. Walbeek, Detection of mycotoxins by thin-layer chromatography: application to screening of fungal extracts, Appl. Microbiol. 20 (1970) 839–842.
[245] A. Malachova, ´ M. Str´ ansk´ a, M. Vaclavíkov ´ ´ a, C.T. Elliott, C. Black, J. Meneely,
J. Hajslova, C.N. Ezekiel, R. Schuhmacher, R. Krska, Advanced LC-MS-based methods to study the co-occurrence and metabolization of multiple mycotoxins in cereals and cereal-based food, Anal. Bioanal. Chem. 410 (2018) 801–825.
[246] S.J. Hird, B.P.Y. Lau, R. Schuhmacher, R. Krska, Liquid chromatography-mass spectrometry for the determination of chemical contaminants in food, TrAC
Trends Anal. Chem. 59 (2014) 59–72.
[247] C.D. Liao, J.W. Wong, K. Zhang, P. Yang, J.B. Wittenberg, M.W. Trucksess, D.
G. Hayward, Multi-mycotoxin analysis of finished grain and nut products using ultrahigh-performance liquid chromatography and positive electrospray ionization-quadrupole orbital ion trap high-resolution mass spectrometry,
J. Agric. Food Chem. 63 (2015) 8314–8332.
[248] L. Marianne, D.B. Siegrid, L. Ben, R. Michael, Siska, D. Mathias, Multi LC-MS/MS and LC-HRMS methods for determination of 24 mycotoxins including major phase
I and II biomarker metabolites in biological matrices from pigs and broiler chickens, Toxins 11 (2019) 171.
[249] L. Righetti, G. Paglia, G. Galaverna, C. Dall’Asta, Recent advances and future challenges in modified mycotoxin analysis: why HRMS has become a key instrument in food contaminant research, Toxins 8 (2016) 361.
[250] C. Luigi, G. Luana, I. Josefa, Target analysis and retrospective screening of multiple mycotoxins in pet food using UHPLC-Q-Orbitrap HRMS, Toxin 11 (2019)
434.
[251] N. Jo˜ ao, C. Catarina, M. Judit, R. Jo˜ ao, A.P. Sofi, M.M.A. Alexandra, Mass spectrometry-based methodologies for targeted and untargeted identification of protein covalent adducts adductomics: current status and challenges,
HighThroughput 8 (2019) 9.
[252] N. Abouseada, M. Raouf, E. EI-Attar, P. Moez, Matrix-assisted laser desorption ionisation time-of-flight mass spectrometry rapid detection of carbapenamase activity in Acinetobacter baumannii isolates, Ind. J. Med. Microbiol. 35 (1) (2017)
85–89.
[253] L. Tuuli, J. Alexandra, M.B. Scott, M.H. Ira, Genomic analysis in the age of human genome sequencing, Cell 177 (2019).
[254] M.A. Mahmoud, Detection of Aspergillus flavus in stored peanuts using real-time
PCR and the expression of aflatoxin genes in toxigenic and atoxigenic A. Flavus isolates, Foodborne Pathog. Dis. 12 (2015) 289–296.
[255] P. Jongsun, L. Woochan, Z. Bohan, M. Anbazhagan, H. In-Beom, K. Jong-Hwa,
H. Hap-Hoon, et al., Complete mitochondrial genome sequence of an aflatoxin B and g producing fungus, Aspergillus parasiticus, Mitochondrial Dna Part B 4 (2019)
947–948.
[256] J. Zhang, R. Chiodini, A. Badr, G. Zhang, The impact of next-generation sequencing on genomics, J. Genet. Genom. 38 (2011) 95–109.
[257] G.G. Moore, B.M. Mack, S.B. Beltz, Genomic sequence of the aflatoxigenic filamentous fungus Aspergillus nomius, BMC Genomics 16 (2015) 551.
[258] G.M. Geromy, M.M. Brian, B.B. Shannon, P. Olivier, Genome sequence of an aflatoxigenic pathogen of argentinian peanut, Aspergillus arachidicola, BMC
Genomics 19 (2018) 189.
[259] T. Takahito, H. Saho, Y. Satoe, T. Hiroki, K. Daisuke, T. Masahiko, D. Kondoh,
Comparative genome analysis of Aspergillus flavus clinically isolated in Japan,
Dna Res. 26 (2018) 95–103.
[260] P.C. Faustinelli, X.M. Wang, E.R. Palencia, R.S. Arias, Genome sequences of eight
Aspergillus flavus spp. and One A. parasiticus sp. isolated from Peanut Seeds in
Georgia, Genome Announcment 4 (2016).
[261] M.K. Gilbert, B.M. Mack, G.A. Payne, D. Bhatnagar, Use of functional genomics to assess the climate change impact on Aspergillus flavus and aflatoxin production,
World Mycotoxin J. 9 (2016) 665–672.
[262] A. Chakrabortti, J. Li, Z.X. Liang, Complete genome sequence of the filamentous fungus Aspergillus westerdijkiae reveals the putative biosynthetic gene cluster of ochratoxin a, Genome Announc. 4 (2016).
[263] X. Han, A. Chakrabortti, J. Zhu, Z.X. Liang, J. Li, Sequencing and functional annotation of the whole genome of the filamentous fungus Aspergillus westerdijkiae, BMC Genomics 17 (2016) 633.
[264] L. Boqiang, C. Yong, Z. Yuanyuan, S. Yanjiao, Z. Zhanquan, X. Xiaodi, et al.,
Dissection of patulin biosynthesis, spatial control and regulation mechanism in
Penicillium expansum, Environ. Microbiol. 21 (2019) 1124–1139.
[265] S. Sayanthooran, D.N. Magana-Arachchi, L. Gunerathne, T. Abeysekera, Potential diagnostic biomarkers for chronic kidney disease of unknown EtiologyCKDu in Sri
Lanka: a pilot study, BMC Nephrol. 18 (2017) 31.
[266] J. Vercruysse, M. Van Bel, C.M. Osuna-Cruz, S.R. Kulkarni, V. Storme, H. Nelissen,
N. Gonzalez, Comparative transcriptomics enables the identification of functional orthologous genes involved in early leaf growth, Plant Biotechnol. J. 18 (2019)
553–567.
[267] N.S. Christine, J.C. Benjamin, C. Doina, L. Junyan, Y. Yuko, G. Mona, M. Gaina,
High-throughput single-cell transcriptome profiling of plant cell types, Cell Rep.
27 (7) (2019) 2241–2247.
[268] R. Lowe, N. Shirley, M. Bleackley, S. Dolan, T. Shafee, Transcriptomics technologies, PLoS Comput. Biol. 13 (2017), e1005457.
[269] G. Castella, ´ M.R. Bragulat, L. Puig, W. Sanseverino, F.J. Cabanes, ˜ Genomic diversity in ochratoxigenic and non ochratoxigenic strains of Aspergillus carbonarius, Sci. Rep. 8 (2018) 5439.
[270] B.M. Musungu, D. Bhatnagar, R.L. Brown, G.A. Payne, G. OBrian, A.M. Fakhoury,
G. Matt, A network approach of gene Co-expression in the Zea mays/aspergillus flavusPathosystem to map Host/Pathogen interaction pathways, Front. Genet. 7 (2016) 206.
[271] S.N. Nayak, G. Agarwal, M.K. Pandey, H.K. Sudini, A.S. Jayale, S. Purohit,
A. Desai, Aspergillus flavus infection triggered immune responses and hostpathogen cross-talks in groundnut during in-vitro seed colonization, Sci. Rep. Ist.
Super. Sanita 7 (2017) 9659.
[272] D. Bhatnagar, K. Rajasekaran, M. Gilbert, J.W. Cary, N. Magan, Advances in molecular and genomic research to safeguard food and feed supply from aflatoxin contamination, World Mycotoxin J. 11 (2018) 47–72.
[273] W. Bo, K. Vivek, O. Andrew, W. Doreen, Reviving the transcriptome studies: an insight into the emergence of single-molecule transcriptome sequencing, Front.
Genet. 10 (2019) 384.
[274] J.C. Fountain, J. Koh, L. Yang, M.K. Pandey, S.N. Nayak, P. Bajaj, Z. Wei-Jian, et al., Proteome analysis of Aspergillus flavus isolate-specific responses to oxidative stress in relationship to aflatoxin production capability, Sci. Rep. 8 (2018) 3430.
[275] X. Xia, H. Li, F. Liu, Y. Zhang, Q. Zhang, Y. Wang, P. Li, Proteome changes in
Penicillium expansum grown in a medium derived from host plant, J. Microbiol.
Biotechnol. 27 (2017) 624–632.
[276] T. Lai, Y. Wang, Y. Fan, Y. Zhou, Y. Bao, T. Zhou, The response of growth and patulin production of postharvest pathogen Penicillium expansum to exogenous potassium phosphite treatment, Int. J. Food Microbiol. 244 (2017) 1–10.
[277] F. Degola, F. Bisceglie, M. Pioli, S. Palmano, L. Elviri, G. Pelosi, T. Lodi, et al.,
Structural modification of cuminaldehyde thiosemicarbazone increases inhibition specificity toward aflatoxin biosynthesis and sclerotia development in Aspergillus flavus, Appl. Microbiol. Biotechnol. 101 (2017) 6683–6696.
[278] P.H. O’Farrell, High-resolution two-dimensional electrophoresis of proteins,
J. Biol. Chem. 250 (1975) 4007–4021.
[279] M. Mohammadi, V. Anoop, S. Gleddie, L.J. Harris, Proteomic profiling of two maize inbreds during early gibberella ear rot infection, Proteomics 11 (2011)
3675–3684.
[280] A. Bilal, B. Madiha, A.N. Muhammad, K. Mohsin, H.R. Muhammad, Proteomics: technologies and their applications, J. Chromatogr. B Biomed. Sci. Appl. 55 (2) (2017) 182–196.
[281] P.W. Alex, D. Wendiro, P.C. Vuzi, J.F. Hawumba, Methods for detection of aflatoxins in agricultural food crops, J. Appl. Chem. (2014) 1–15.
[282] I.Y. Goryacheva, S. De Saeger, S.A. Eremin, C. Van Peteghem, Immunochemical methods for rapid mycotoxin detection: evolution from single to multiple analyte screening. A review, Food Add. Cont. (2007) 1169–1183.
[283] N. Saraf, Development of in vitro point of care diagnostics (IVPCD) based on aptamers integrated biosensors, Electronic (2019) 6618.
[284] K. Sefah, et al., In vitro selection with artificial expanded genetic information systems, Proc. Natl. Acad. Sci. U. S. A. 111 (2014) 1449–1454.
[285] R. Bazaz, D.W. Denning, Aspergillosis: causes, types, and treatment, Pharm. J.
303 (2019) 7927, https://doi.org/10.1211/PJ.2019.20206738.
[286] R. Malcolm, R. Riina, Exposure to Aspergillus in home and healthcare facilities’ water environments: focus on biofilms, Microorganisms 7 (2019) 7, https://doi. org/10.3390/microorganisms7010007.
[287] R.P. Vivek-Ananth, K. Mohanraj, M. Vandanashree, A. Jhingran, P. James Craig,
Areejit Samal, Comparative systems analysis of the secretome of the opportunistic pathogen Aspergillus fumigatus and other Aspergillus species, Sci. Rep. (2017)
1–31.
[288] N. Raffa, Nancy P. Keller, A call to arms: mustering secondary metabolites for success and survival of an opportunistic pathogen, PLoS Pathog. 15 (2019) 4.
[289] H. Salah, M. Lackner, J. Houbraken, B. Theelen, C. Lass-Florl, ¨ T. Boekhout,
M. Almaslamani, S.L. Taj-Aldeen, The emergence of rare clinical Aspergillus species in Qatar: molecular characterization and antifungal susceptibility profiles,
Front. Microbiol. 10 (2019) 1677.
[290] P.D. Barnes, K.A. Marr, Aspergillosis: spectrum of disease, diagnosis, and treatment, Infect. Dis. Clin. N Am. 20 (2006) 545–561.
[291] T. Oguma, M. Taniguchi, T. Shimoda, K. Kamei, H. Matsuse, A. Hebisawa,
N. Takayanagi, S. Konno, K. Fukunaga, K. Harada, J. Tanaka, K. Tomomatsu,
K. Asano, Allergic bronchopulmonary aspergillosis in Japan: a nationwide survey,
Allergol. Int. 67 (2018) 79–84.
[292] B. Nepal, R. Myers, J.M. Lohmar, O. Puel, B. Thompson, M. Van Cura, A.M. Calvo,
Characterization of the putative polysaccharide synthase CpsA and its effects on the virulence of the human pathogen Aspergillus fumigatus, PLoS One (2019)
1–18.
[293] A.R.C. Luis, P.T.O. Ana, L.A.R. Jos´e, P. Bayman, An opportunistic human pathogen on the fly: strains of Aspergillus flavus vary in virulence in Drosophila melanogaster, Med. Mycol. 52 (2014) 211–219.
[294] M.J. Amiri, P. Karami, A.H. Chichaklu, E.H. Jangan, Identification and isolation of Mycobacterium tuberculosis from Iranian patients with recurrent TB using different staining methods, J Res. Med. Dent Sci. 6 (2018) 409–414.
[295] S. Sivasankari, et al., Prevalence of Invasive Aspergillosis Among (PTB) Patients in Kanchipuram, India, J. Clin. Diagn. Res. 8 (2014) 22–23, https://doi.org/
10.7860/JCDR/2014/7957.4094.
[296] L.D. Page, Pulmonary Aspergillosis in Association With Tuberculosis and HIV in
Uganda, School of Medicine / Institute of Inflammation and Repair, 2021.
[297] R. Hernandez-Martínez, ´ I. Navarro-Blasco, Aflatoxin levels and exposure assessment of Spanish Infant Cereals, Food Addit. Contam.: Part B. Surveill. 3 (4) (2010) 275–288.
[298] G.D. Dorr, Y. Iwona, W.G. Sorenson, J.M. Martha, A.E. Ruth, Overview of investigations into pulmonary haemorrhage among infants in Cleveland, Ohio,
Environ. Health Perspect. 107 (1999) 495–499.
[299] W. Jedrychowski, U. Maugeri, F. Perera, L. Stigter, J. Jankowski, M. Butscher,
B. Maria, et al., Cognitive function of 6-year-old children exposed to moldcontaminated homes in early postnatal period. Prospective birth cohort study in
Poland, Physiol. Behav. 104 (2011) 989–995.
[300] S.J. Allen, C.P. Wild, J.G. Wheeler, E.M. Riley, R. Montesano, S. Bennett,
Aflatoxin exposure, malaria and hepatitis B infection in rural Gambian children,
Trans. R. Soc. Trop. Med. Hyg. 86 (4) (1992) 426–430.
[301] A. Oloyede, A. Olusegun, R. Assunta, S. Yinka, B. Ranajit, R. Alberto, Correlation between aflatoxin M1 content of breast milk, dietary exposure to aflatoxin B1 and socioeconomic status of lactating mothers in Ogun State, Nigeria, Food Chem.
Toxicol. 56 (2013) 171–177.
[303] O. Puel, P. Galtier, P.I. Oswald, Biosynthesis and toxicological effects of patulin,
Toxins (2010) 2.
[304] H. Hajjaj, A. Klaebe, M.O. Loret, G. Goma, P.J. Blanc, J.F. Francois, Biosynthetic pathway of citrinin in the filamentous fungus Monascusruber as revealed by 13C nuclear magnetic resonance, Appl. Environ. Microbiol. (1999) 311–314.
[305] Y.V.V. Aswani Kumar, R.M. Renuka, J. Achuth, M. Venkataramana,
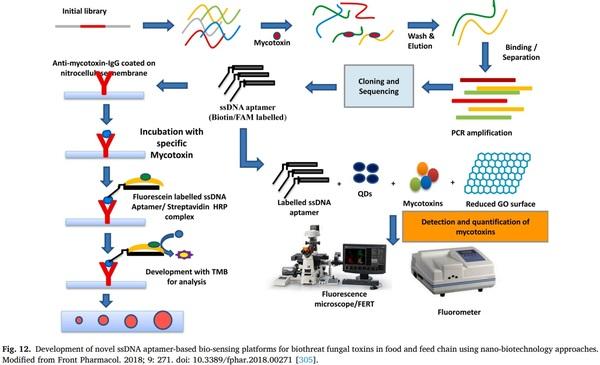
M. Ushakiranmayi, P. Sudhakar, Development of hybrid IgG-Aptamer sandwich immunoassay platform for aflatoxin B1 detection and its evaluation onto various field samples, Front. Pharmacol. 9 (2018) 271, https://doi.org/10.3389/ fphar.2018.00271.






.jpg&w=3840&q=75)