In vitro gas production of fibrous substrates with the inclusion of yeast
Author details:
1 Instituto de Ciencia Animal, Apartado Postal 24, San José de las Lajas, Mayabeque, Cuba; 2 Universidad Autónoma de Chihuahua, Periférico Francisco R. Almada, km 1 CP 31453. Chihuahua, Chih, México; 3 Univeridad Autónoma de Ciudad Juárez, División Multidisciplinaria en Nuevo Casas Grandes de la Universidad Autónoma de Ciudad Juárez. Av. Universidad 3003, Sección Hidalgo. C.P: 31803, Chihuahua, México; 4 Centro Universitario de Guantánamo, Carretera a Santiago de Cuba, km 2.5, Guantánamo, Cuba.
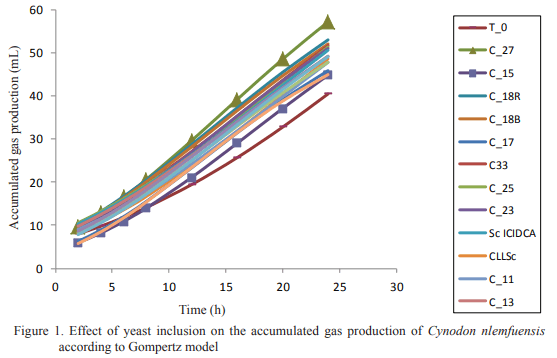
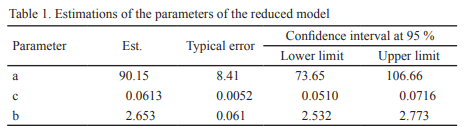
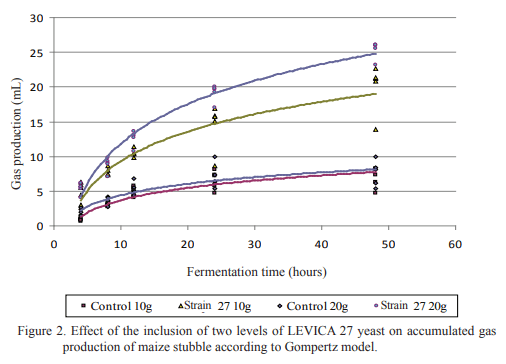
The in vitro gas production technique was applied for studying the effect of the yeast species and its inclusion dosage on ruminal fermentation of fibrous substrates. Two experiments were executed. In the first, the effect of yeast inclusion of different species on in vitro gas production of star grass (Cynodon nlemfuensis) was determined and in the second, two levels (5 and 10 mg of DM/mL) of the yeast strain showing the best performance in the first experiment were included for confirming its effect on gas production of maize stubble. Controls without yeast and a blank with the non-inoculated culture were included. As result, all strains had a gas production higher than the control, except in that including strain 15. The best performance was in the one in which strain 27 was incorporated. Strains of S. cerevisiae had an intermediate performance regarding the rest. The inclusion of 10 mg DM/mL of the strain 27 (Pichia guillermondii) stimulated in vitro gas production of the maize stubble until 48 h of fermentation. It is concluded that the species and strain of yeasts, as well as their inclusion dosage, have determinant effect on in vitro gas production of different fibrous substrates. This reasserts the importance of selecting the adequate strains for their utilization as additive in diets for ruminants, according to the feed intended to be used.
Key words: yeasts, Pichia guillermondii, rumen, gas production


Balzarini, M. G., González, I., Tablada, M., Casanoves,F., Di Rienzo, J. A. & Robledo, C. W. 2012. Paquete estadístico Infostat. Universidad Nacional de Córdoba, Argentina
Beauchemin, K. A., Yang, W. Z., Morgavi, D. P., Ghorbani, G. R., Kautz, W. & Leedle, J. A. Z. 2003. Effects of bacterial direct-fed microbials and yeast on site and extent of digestion, blood chemistry, and subclinical ruminal acidosis in feedlot cattle. J. Anim. Sci. 81:1628
Castillo, Y. 2009. Fermentación in vitro para obtener la levadura Candida norvegensis en mezclas de alfalfa y paja de avena con bagazo de manzana fermentado y sus efectos sobre la actividad microbiana ruminal. PhD Thesis. Facultad de Zootecnia y Ecología. Universidad Autónoma de Chihuahua. Chihuahua
Chaucheyras-Durand, F., Walker, N.D. & Bach, A. 2008. Effects of active dry yeasts on the rumen microbial ecosystem: Past, present and future. Anim. Feed Sci. Technol. 14:5.
Combellas, J., Jacqueline, S., Tesorero, M. & Gabaldón, L. 2002. Animal response of yearling cattle to the addition of yeast culture to a diet of pasture, poultry litter and wheat middlings. Zootecnia Trop. 20:373.
De Ondarza, M.B., Sniffen, C.J., Dussert, L., Chevaux, E. & Sullivan, J. 2011. Live yeast aids rumen function, milk yield. Feedstuffs. Reprinted with permission from. 83:24
Doleža, P., Dvorácek,J., Doležal.J., Cermáková, J., Zeman,L. & Szwedziak, K.2011. Effect of feeding yeast culture on ruminal fermentation and blood indicators of Holstein dairy cows. Acta Vet. BRNO. 80: 139
Enjalbert, F., Garrett, J.E., Moncoulon, R., Bayourthe, C., & Chicoteau, P. 1999. Effects of yeast culture (Saccharomyces cerevisiae) on ruminal digestion in non-lactating dairy cows. Animal Feed Sci. Tech. 76:195
Galindo, J., Marrero, Y., González, N., Sosa, A., Miranda, A.L., Aldana, A.I., Moreira, O., Bocourt, R., Delgado, D., Torres, V., Sarduy, L. & Noda, A. 2010. Effect of preparations with the viable yeasts Saccharomyces cerevisiae and LEVICA-25 on methanogens and in vitro ruminal methanogenesis. Cuban J. Agric. Sci. 44:267
Galindo, J., Torres, V., Rodríguez, Z., Álvarez, E., Aldana, A.I., Rodríguez, R., García, R., Boucourt, R., Delgado, D., Martín, E., Moreira, O., Noda, A., Núñez, O., González, M., Herrera, F., Cairo, J. & Rodríguez, F. 2008. Avances en el estudio de las levaduras como activadoras de la fermentación ruminal en bovinos que consumen dietas fibrosas. Ciencia en la Frontera. Rev de Cienc. y Tec. de la UACJ. VI. Número especial: 93
Jay, O., Torres, V., Marrero, Y. & Torres, J.P. 2012a. Tests assessements for multiple comparisons of in vitro gas curves from the root of the mean square distance. Cuban J. Agric. Sci. 46:133
Jay, O., Torres, V., Marrero, Y. & Torres, J.P. 2012b. Sensibility analysis of homogencity tests of in vitro gas production curves by Monte Carlo simulation. Cuban J. Agric. Sci. 46:15-22.
Lee, J.H., Lim, Y.B., Koh, J.H., Baig, S.Y. & Shin, H.T. 2002. Screening of thermotolerant yeast for use as microbial feed additive. J. Microbiol. Biotechnol.12:162
Marrero, Y., Castillo, Y., Burrota, E., Lovaina, T., Rosa, C.A., Ruiz, O., González, E. & Basso, LC. 2011. Morphological, biochemical, and molecular identification of the yeast Levica 25: a potential ruminal microbial additive. Global Veterinaria 7:60
Marrero, Y., Burrola, M.E., Castillo,Y., Basso, L.C., Rosa, C.A.; Ruiz, O., González, E. 2013. Identification of Levica yeasts as a potential ruminal microbial additive. Czech J. Anim. Sci. 10: 460
Montgomery, D.C. 2004. Diseño y análisis de experimentos. Limusa-Wiley. México, D.F. p.692
Newbold, C.J., McIntosh, F.M., & Wallace RJ. 1996. Mode of action of the yeast, Saccharomyces cerevisiae, as a feed additive for ruminants. Br. J. Nutr. 76:249.
Olson, K.C., Caton, J.S., Kirby, D.R. & Norton, P.L. 1994. Influence of yeast culture supplementation and advancing season on steers grazing mixed-grass prairie in the northern great plains: II. Ruminal fermentation, site of digestion, and microbial efficiency. J. Anim. Sci. 72: 2158.
Rojo, R., Mendoza, G.D., García, C. M., Bárcena, J. R. & Aranda, E. M. 2000. Intake and digestibility of tropical grasses in steers with nitrogen supplementation and Saccharomyces cerevisiae . Rev. Fac. Agron (LUZ). 17: 358
Sánchez, M.I., Santos, A., Dustet, J.C., Guerra, G., León, T., Argüelles, J., Ramos-Leal, M., Margarita, A., Casado, G., & Gómez, B. 2007. Estudio fisiológico de una cepa de levadura con potencialidades para el enriquecimiento proteico del bagazo de caña de azúcar. Revista CENIC Ciencias Biológicas. 38: 39
Stella, A.V., Paratte, R., Valnegri, L., Cigalino, G., Soncini, G., Chevaux, E., Dell’Orto, V. & Savoini, G. 2007.Effect of administration of live Saccharomyces cerevisiae on milk production, milk composition, blood metabolites, and faecal flora in early lactating dairy goats, Small Rumin. Res. 67:7.
Sullivan, H.M. & Martin, S.A. 1999. Effects of Saccharomyces cerevisiae cultures on in vitro mixed ruminal microorganism fermentation. J. Dairy Sci. 82: 2011.
Tang, S.X., Tayo, G.O., Tan, Z.L., Sun, Z.H., Shen, L.X., Zhou, C.S., Xiao, W.J., Ren, G.P., Han, X.F. & Shen, S.B. 2008. Effects of yeast culture and fibrolytic enzyme supplementation on in vitro fermentation characteristics of low-quality cereal straws. J. Anim. Sci. 86: 1164.
Theodorou, M.K., Williams, B.A., Dhanoa, M.S., McAllan, A.D.B. & France, J. 1994. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds, Anim Feed Sci. Technol. 48: 185
Thornley, J.H.M. & France, J. 2007. Mathematical Models in Agriculture. CAB International. Oxon. p.889
Generally, cereals may not affect rumen & mostly legumes based have the state of impaction. The experiment needs to be elevated to next level.








.jpg&w=3840&q=75)