The Effects of Lactic Acid Bacterial Inoculants on The Fermentation, Aerobic Stability and Nutritive Value of Sunflower Silages
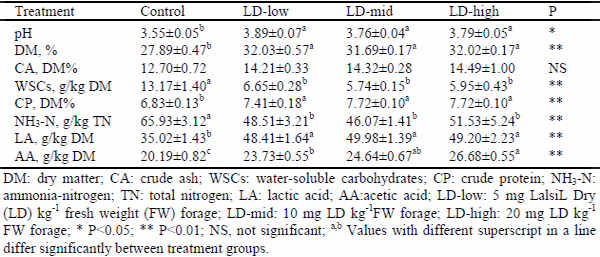
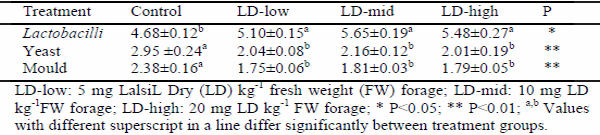
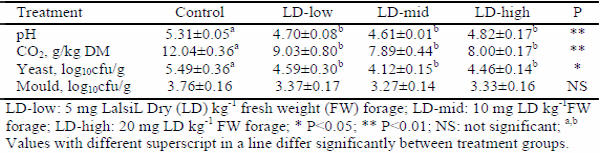
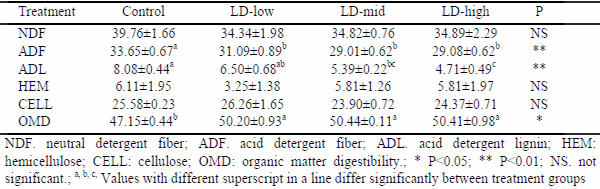
This study was implemented to determine the effect of lactic acid bacterial inoculants as silage additives on fermentation, aerobic stability, and in vitro organic matter digestibility of sunflower silages. Lalsil®Dry (Lallemand, France), containing wáter soluble Lactobacillus buchneri, Pediococcus acidilactici with cellulase and hemicellulase enzymes, was chosen as bacterial inoculants. The inoculants were applied to silages at the rates of 5, 10 and 20 mg kg-1 fresh weight levels. After the treatment, chopped whole crop sunflower was ensiled in 1.0-L special vacuum bags. The bags were stored at 25±2 °C under laboratory conditions. Three bags from each group were sampled for chemical and microbiological analysis on the 90th day after ensiling. At the end of the ensiling period, all silages were subjected to an aerobic stability test for five days. In addition, in vitro organic matter digestibility of all silages was determined. The results showed that lactic acid bacterial inoculants enhanced the characteristics of fermentation and decreased acid detergent fiber and acid detergent lignin contents of sunflower silages. When compared to the control group, the aerobic stability was found to get improved in Lalsil treatments, as indicated by reduced pH value, carbon dioxide production and yeast populations. Treated silage groups appeared with higher in vitro organic matter digestibility than the control group.
Key words: Sunflower silage, Fermentation, Aerobic stability, Nutrition value.
Aksu, T., Baytok, E., Bolat, D. 2004. Effects of a bacterial silage inoculant on corn silage fermentation and nutrient digestibility. Small Ruminant Research, 55: 249-252.
Akyildiz, A.R., 1986. Yemler bilgisi ve teknolojisi. Ankara Üniversitesi. ZiraatFakültesi Yayinlari, Yayin No: 974, Ders Kitabi No:286, Ankara.
Anonymus, 1986. The Analysis of Agricultural Material. Reference Book.pp. 427-428. London.
AOAC, 1990. Official Methods of Analysis. 15th ed.. Association of Official Analytical Chemists. Arlington. Virginia. USA.
Ashbell, G., Weinberg, Z.G., Azrieli, A., Hen, Y.,Horev, B. 1991. A simple system to study the aerobic deterioration of silages. Canadian Agriculture Engineering, 33: 391-393.
Aufrère, J., Michalet-Doreau, B., 1988. Comparison of methods for predicting digestibility of feeds. Animal Feed Science and Technology, 20:203-218.
Basso, F. C., Adesogan, A. T., Lara, E. C., Rabelo, C. H. S., Berchielli, T. T., Teixeira, I. A. M. A. G. , Siqueira, R., and Reis R. A., 2014. Effects of feeding corn silage inoculated with microbial additives on the ruminal fermentation, microbial protein yield, and growth performance of lambs. Journal of Animal Science, 92:5640–5650.
Basso, F.C., Bernardes, T.F., Roth A.P.T.P., Lodo B.N., Berchielli T.T., Reis, R.A., 2012. Fermentation and aerobic stability of corn silage inoculated with Lactobacillus buchneri.Revista Brasileira de Zootecnia, 41(7):1789- 1794.
Demirel, M., Bolat, D., Celik, S., Bakici, Y.andEratak, S., 2008. Determination of fermentation and digestibility characteristics of corn, sunflower and combination of corn and sunflower silages. Journal of Animal and Veterinary Advences, 7:707-711
Demirel, M.,Bolat, D.,Çelik, S.,Bakici, Y., Çelik, S. 2006. Quality of silages from sunflower harvested at different vegetational stages. Journal of Applied Animal Research, 30:161-165.
Driehuis, F., Elferink, S. and Spoelstra, S., 1999. Anaerobic lactic acid degradation during ensilage of whole crop maize inoculated with Lactobacillus buchneri inhibits yeast growth and improves aerobic stability. Journal of Applied Microbiolgy, 87:583-594.
Driehuis, F., Oude Elferink, S. J. W. H., Van Wikselaar, P. G., 2001. Fermentation characteristics and aerobic stability of grass silage inoculated with Lactobacillus buchneri, with or without homofermentative lactic acid bacteria. Grass and Forage Science, 56(4):330-343.
Driehuis, F., Wikselaar, Van P.G., Van Vuuren, A.M., Spoelstra, S.F., 1997. Effect of a bacterial inoculant on rate of fermentation and chemical composition of high dry matter grass silages. Journal of AgriculturalScience, 128:323-329.
Filya, I. 2003b. The effect of Lactobacillus buchneri with or without homofermentative lactic acid bacteria. on the fermentation. aerobic stability and ruminal degradability of wheat. sorghum and maize silages. Journal of Applied Microbiology, 95:1080–1086.
Filya, I., 2000. Silaj kalitesinin arttirilmasinda yeni gelismeler. International Animal Nutrition Congress’2000. p. 243-250. Isparta.
Filya, I., 2003a. The effect of Lactobacillus buchneri and Lactobacillus plantarum on the fermentation. aerobic stability. and ruminal degradability of low dry matter corn and sorghum silages. Journal of Dairy Science, 86:3575- 3581.
Filya, I., Sucu, E. and Karabulut, A., 2006. The effect of Lactobacillus buchneri on the fermentation, aerobic stability and ruminal degradability of maize silage. Journal of Applied Microbiology, 101: 1216-1223.
Filya, I., Sucu, E., 2007. The effect of bacterial inoculants and a chemical preservative on the fermentation and aerobic stability of whole-crop cereal silages. Asian-Aust. Journal of Animal Science, 20(3):378-384.
Gandra, J.R., Oliveira, E.R., Gandra, E.R.S., Takiya, C.S., Goes, R.H.T.B., Oliveira, K.M.P., Silveira, K.A., Araki, H.M.C., Orbach, N.D., Vasquez, D.N. 2017. Inoculation of Lactobacillus buchneri alone or with Bacillus subtilis and total losses, aerobic stability, and microbiological quality of sunflower silage, Journal of Applied Animal Research, 45:1, 609-614.
Goering, H.K., Van Soest, P.J. 1983. Forage Fiber Analyses. Agricultural Handbook. No 379. Washington.
Goes, R.H.T.B., Cerilo, S.L.N., Lima, H.L., Fernandes, A.R.M., Oliveira, E.R., Souza, K.A., Patussi, R.A., Brabes, K.C.S., Gressler, M.G.M., 2012. Torta de girassol em substituição ao farelo de soja nos suplementos de novilhas: desempenho e características de carcaça. Revista Brasileira de Saúde e Produção Animal, 13:396–409.
Hargreaves, A., Hill, J., Leaver, J.D., 2009. Effect of stage of growth on the chemical composition, nutritive value and ensilability of whole-crop barley.Animal Feed Science and Technology, 152:50–61.
Harrison, J.H., Soderlund, S.O., Loney, K.A., 1989. Effect of inoculation rate of selected strains of lactic acid bacteria on fermentation and in vitro digestibility of grass-legume forage. Journal of Dairy Science, 72 (9):2421-2426.
Holzer, M., Mayrhuber, E., Danner, H., Braun, R. 2003. The role of Lactobacillus buchneri in forage preservation. Trends Biotechnology, 21:282–287.
Hu, W., Schimdt, R.J., McDonell, E.E., Klingerman, C.M., Kung, Jr. K. 2009. The effect of Lactobacillus buchneri 40788 or Lactobacillus plantarum MTD-1 on the fermentation and aerobic stability of corn silages ensiled at two dry matter contents. Journal of Dairy Science, 92:3907–3914.
Kleinschmit, D.H., Kung, Jr. L.A., 2006. A meta-analysis of the effects of Lactobacillus buchneri on the fermentation and aerobic stability of corn and grass and small-grain silages. Journal of Dairy Science, 89:4005–4013.
Koc, F., Coskuntuna, L. 2003. Silo yemlerinde organic asit belirlemede iki farkli metodun karsilastirilmasi. Journal of Animal Production, 44:37-47.
Koc, F., Ozduven, M.L., Coskuntuna, L., Polat, C. 2009. The effects of inoculant lactic acid bacteria on the fermentation and aerobic stability of sunflower silage. Poljoprivreda / Agriculture, 15(2):47-52.
Kristensen, N.B., Sloth, K.H., Hojberg, O., Spliid, N.H., Jensen, C. and Thogersen, R. 2010. Effects of microbial inoculants on corn silage fermentation, microbial contents, aerobic stability and milk production under field conditions. Journal of Dairy Science, 93:3764-3774.
Kung, L., Ranjit, N. K. 2001. The effect of Lactobacillus buchneri and other additives on the fermentation and aerobic stability of barley silage. Journal of Dairy Science, 84(5), 1149- 1155.
Kung, L., Shaver, R., 2001. Interpretation and use of silage fermentation analysis reports. Focus on Forage, 3:1-5.
Li, P., Bai, S., You, M., Shen, Y. 2016a. Effects of maturity stage and lactic acid bacteria on the fermentation quality and aerobic stability of Siberian wildrye silage. Food Science and Nutrition, 4(5): 664–670.
Li, X., Xu, W., Yang J., Zhao H., Pan C., Ding X., Zhang, Y. 2016b. Effects of applying lactic acid bacteria to the fermentation on a mixture of corn steep liquor and air-dried rice straw. Animal Nutrition, 2: 229-233.
McDonald, P., Edwards, R.A., Greenhalgh, J.F.D., Morgan, C.A. 2002. Animal Nutrition. 6th Edition. Longman Scientific and Technical. pp. 515-535.
McDonald, P., Henderson, A.R., Heron, S.J.E., 1991. The Biochemistry of Silage. Chalcombe Publications. 13 Highwoods drive. Marlow Bottom. Marlow. Bucks. UK.
Mohammadzadeh, H., Khorvash, M., Ghorbani, G.R., Yang, W.Z. 2011. Effects of a dual-purpose bacterial inoculant on the fermentation characteristics of high-moisture maize silage and dairy cattle performance. South African Journal of Animal Science, 41 (4): 368-376.
Nkosi, B.D., Meeske, R., Langa, T., Motiang, M.D., Mutavhatsindi, T.F., Thomas, R.S., Groenewald, I.B., Baloyi, J.J. 2015. The influence of ensiling potato hash waste with enzyme/bacterial inoculant mixtures on the fermentation characteristics, aerobic stability and nutrient digestion of the resultant silages by rams. Small Ruminant Research, 127: 28–35.
Nkosi, B.D., Meeske, R., Langa, T., Motiang, M.D., Modiba, S., Mutavhatsindi, T.F., Malebana, I.M.M., Groenewald, I.B. 2016. Effects of bacterial inoculation on the fermentation characteristics and aerobic stability of ensiled whole plant soybeans (Glycine max (L.) Merr.). South African Journal of Animal Science, 46 (2):129-138.
Nkosi, B.D., Vadlani, P.V., Brijwani, K., Nanunja, A., Meeske, R. 2012. Effects of bacterial inoculants and an enzyme on the fermentation quality and aerobic stability of ensiled whole crop sweet sorghum. South African Journal of Animal Science,42:232-240.
Nkosi, B.D.,Meeske, R. 2010. Effects of ensiling totally mixed potato hash ration with or without a heterofermentative bacterial inoculant on silage fermentation, aerobic stability, growth performance and digestibility in lambs. Animal Feed Science and Technology, 161, 38-48.
Ozduven, M. L., Koc, F., Polat, C., Coskuntuna, L. 2009. The effects of lactic acid bacteria and enzyme mixture inoculants on fermentation and nutrient digestibility of sunflower silage. The Journal of the Faculty of Veterinary Medicine University of Kafkas, 15 (2): 195-199.
Ozduven, M.L., Koc, F., Akay, V. 2017. Effects of bacterial inoculants and enzymes on the fermentation, aerobic stability and in vitro organic matter digestibility characteristics of sunflower silages. Pakistan Journal of Nutrition, 16: 22-27.
Peiretti, P.G., Meineri, G. 2010. Evolution of chemical composition, nutritive value and fatty acid content of sunflower (Helianthus annuusL.) during the growth cycle. Journal of Animal and Veterinary Advences, 9: 112-117.
Reich, L.J., Kung Jr. L. 2010. Effects of combining Lactobacillus buchneri 40788 with various lactic acid bacteria on the fermentation and aerobic stability of corn silage. Animal Feed Science and Technology, 159:105– 109.
Seale, D.R., Pahlow, G., Spoelstra, S.F., Lindgren, S., Dellaglio, F., Lowe, J.F. 1990. Methods for the microbiological analysis of silage. Proceeding of The Eurobac Conference, 147. Uppsala.
Sheperd, A.C., Maslanka, M., Quinn, D., Kung, L. 1995. Additives containing bacteria and enzymes for alfalfa silage. Journal of Dairy Science,78:565-572. SPSS, 2007. SPSS 15 for Windows. SPSS Inc.
Sucu, E., Aydogan Cifci, E. 2016. Effects of lines and inoculants on nutritive value and production costs of triticale silages. Revista Brasileira de Zootecnia, 45(7):355-364.
Sucu, E., Filya, I. 2006. The effects of bacterial inoculants on the fermentation. aerobic stability and rumen degradability characteristics of wheat silages. Turkish Journal of Veterinary and Animal Sciences, 30:187-193.
Tabacco, E., Piano, S., Cavallarin, L., Bernardes, T.F. and Borreani, G. 2009. Clostridia spore formation during aerobic deterioration of maize and sorghum silages as influenced by Lactobacillus buchneri and Lactobacillus plantarum inoculants. Journal of Applied Microbiology,107: 1632–1641.
Wang, M., Yang, C., Jia L., and Yu K. 2014. Effect of Lactobacillus buchneri and Lactobacillus plantarum on the fermentation characteristics and aerobic stability of whipgrass silage in laboratory silos. Japanese Society of Grassland Science, 60: 233–239.
Weinberg, Z.G., Ashbell, G., Hen, Y., Azrieli A, Szakacs, G. And Filya, I. 2002. Ensiling whole crop wheat and corn in large containers with lactobacillus plantarum and lactobacillus buchneri. Journal of Industrial Microbiology Biotechnology, 28:7-11.
Weinberg, Z.G., Ashbell, G., Hen, Y., Azrieli, A. 1993. The effect of applying lactic acid bacteria at ensiling on the aerobic stability of silages. Journal of Applied Bacteriology, 75:512–518.
Weinberg, Z.G., Ashbell, G., Yaira, H., Azrieli, A. 1995. The effect of cellulase and hemicellulase plus pectinase on the aerobic stability and fiber analysis of peas and wheat silages. Animal Feed Science and Technology, 55: 287-293.
Woolford, M. K. 1990. The detrimental effects of air on silage. Journal of AppliedBacteriology, 68:101-116.
Xu, Ch., Wang, H., Yang, F. and Yu, Zh, 2011. Effect of an inoculant and enzymes on fermentation quality and nutritive value of erect milkvetch (Astragalus adsurgens Pall.) silages. Animal Feed Science and Technology, 20: 449-460.
Zhang, T., Jin, Y.M., Zhang, Y.J., Yu, Z., Yan, W.H. 2013. Silage quality and preservation of Urtica cannabina ensiled alone and with additive treatment. Grass and Forage Science, 69:405–414.
Zhang, T., Li, L., Wang, X.F., Zeng, Z.H., Hu, Y.G., Cui, Z.J. 2009. Effects of Lactobacillus buchneri and Lactobacillus plantarum on fermentation. aerobic stability. bacteria diversity and ruminal degrability of alfalfa silage. World Journal of Microbiology and Biotechnology, 25 (6): 965.








.jpg&w=3840&q=75)