Introduction
Prevention of disease transmission and enhancement of growth and feed efficiency are critical factors in modern pig production. For more than 50 years enteric disease suppression and growth promotion have been effectively achieved by the inclusion of various antibiotics or chemotherapeutics at subtherapeutic doses into diets. The estimated economic benefits in terms of improved growth rates ranged from 3.3 to 8.8% and feed efficiency from 2.5 to 7.0% (Viaene and Verbeke, 1999). However, in recent years more scientific evidence was gathered on a relationship between the feed medication and pathogenic resistance in human therapy (Aarestrup, 2000). In consequence, all in-feed antibiotics and chemotherapeutics will be banned in the EU by 2006. In this context, a particular scientific interest is now paid to the antimicrobial potency of short chain fatty acids (SCFA) and their salts when applied individually or as a mix. This paper outlines some essentials on the physicochemical properties and mode of action of supplemental SCFA and their salts, which in conjunction with endogenous SCFA from particular sections of the gut, can be addressed for suppressing pathogenic growth/colonization and improving digestion, absorption, mucosal immunity, and trophic effects on intestinal brush border (villi and crypts). Besides, the effects of graded doses and sources of SCFA during post-weaning, growing-finishing and reproductive cycles of pigs on performance are presented.
Physicochemical Properties and Mode of Action of SCFA
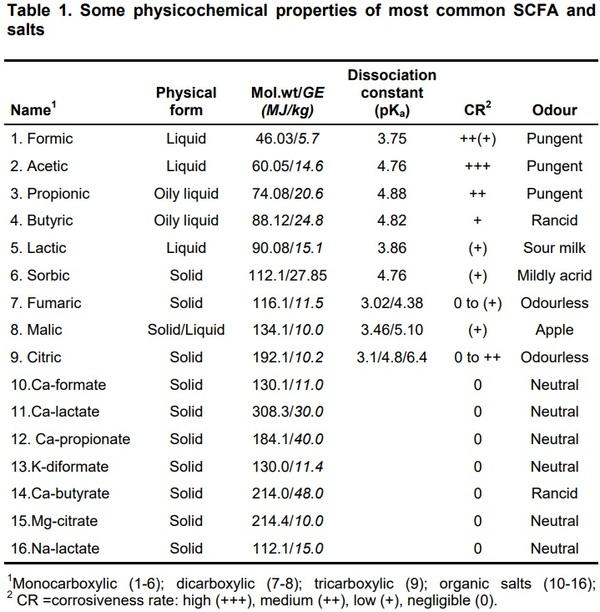
Natural feed resources (fresh, pre-fermented or ensiled ingredients of plant or animal origin and additives) contain more than 100 carboxylic acids and/or derivatives. Among supplemental SCFA and their salts for pigs, a particular practical interest is focused on those listed in Table 1.
Among other acidifiers officially approved in the EU are the following: Nasorbate, Ca-sorbate, K-sorbate, tartaric acid, Na-tartarate, K-tartarate, NaKtartarate, NH3-formate, Na-formate, NH3-propionate, Na-propionate, K-acetate, Ca-acetate, Na-diacetate, Na-citrate, K-citrate, K-lactate, benzoic acid and Na-benzoate. These acidifiers can be administered individually or as a mix (to broaden a spectrum of antimicrobial potency) via feeds or drinking water. The solid acidifiers are easier to handle, whereas the liquid forms may be volatile (up to 20%) during spraying, and their disadvantage could be the corrosiveness and unpleasant odour). This disadvantage can be eliminated by a coating technology for the encapsulation and sustained controlled release taste masking. Moreover, this technology allows also controlling their site of action, as well as the velocity of release and dissociation (Von Felde and Rudat, 1998; Gauthier, 2002).
The mode of action of particular organic acids and salts is not uniform, although a consensus seems to be achieved on the following items:
• that undissociated forms diffuse across cell membranes of pathogens, destroying their cytoplasm or inhibiting growth (inactivation of bacterial decarboxylases and catalases);
• intestinal dissociation liberates H+ ions serving as a pH barrier against pathogen colonisation on the brush border;
• reduced gastric pH in complementariness to endogenous HCl;
• gastric hydrolysis liberates H+ ions activating pepsinogen and inhibiting bacterial growth (bactericidal/bacteriostatic effects)
• energetic substrate or modulator/stimuli for mucosal development, epithelial cell growth and greater absorptive capacity;
• precursors for synthesis of non-essential amino acids, DNA and higher lipids required for intestinal growth;
• increased blood flow and hypocholesterolemic effect.
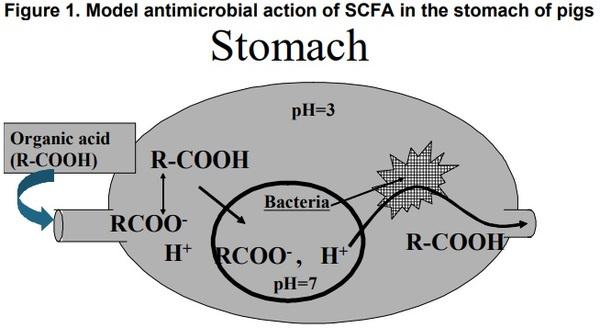
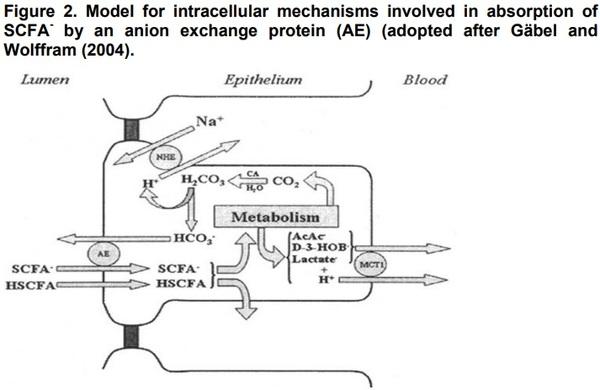
Models for gastric bactericidal action and transepithelial transport mechanisms of SCFA- are presented in Figures 1 and 2, respectively.
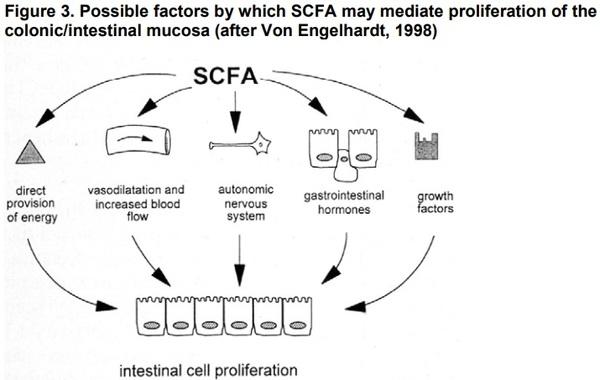
In the post-absorptive phase SCFA may play diverse bio-regulatory roles in controlling mucosal growth, epithelial cell proliferation, apoptosis and regulation of transcriptional proteins, modulation of gene expression, systemic acid-base balance, as well as in whole body energy metabolism and turnover (Figure 3).
In Vitro Evaluation of Antimicrobial Potency of SCFA and Their Salts
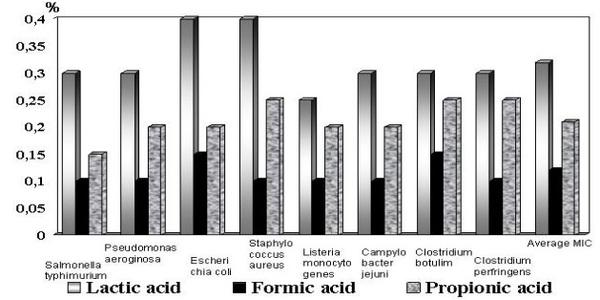
In vitro bactericidal activity of SCFA is measured as their minimum inhibitory concentration (MIC) for target pathogens. The MIC is the concentration of SCFA that will prevent visible growth, either on an agar surface or in liquid culture, after an incubation period. The MIC-values measured for formic, propionic and lactic acids are presented in Figure 4.
Figure 4. Minimum inhibitory concentrations (in %) of SCFA (after Strauss and Hayler, 2001).
Knarreborg et al. (2001) and Naughton & Jensen (2001) investigated the effects of formic, propionic, butyric, lactic, benzoic and fumaric acids, as well as potassium diformate (KDF) on the population changes of coliform and lactic acid bacteria in the gastric digesta at pH 3, 4, and 5, and in the content from the proximal part of the small intestine at pH 5, 6, and 7. These studies demonstrated a clear selective removal of target species, i.e., coliform bacteria from the gastric digesta. Their survival rate was strongly influenced by pH (a regeneration cycle lasted 70 min at pH 5, and 25 min at pH 7), irrespective of the digesta origin. The authors established the following order of killing potency of coliform bacteria: propionic< formic< butyric< lactic< fumaric< benzoic. The latter was superior to the other acids tested in exhibiting a bactericidal effect on coliform as well as lactic acid bacteria, irrespective to the digesta origin. In another study, Jensen et al. (2001) demonstrated that the potency of these acids against Salmonella typhiumurium in gastric digesta at pH4 was in the following order: acetic < formic < propionic < lactic < sorbic < benzoic.
In vivo Production Rate of SCFA in Pigs
In vitro screening of supplemental SCFA is merely indicative, and measurement of their in vivo potency is necessary. They may affect numerous physiological factors (acidity of digesta at specific locations of the digestive tract, epithelial cell proliferation, rate of gastric emptying, digestibility and retention of nutrients, gastrointestinal microflora, pancreatic enzyme secretion), which reflect the pig’s clinical health (incidences of diarrhoea outbreaks, fever, mortality, morbidity) and performance (feed intake, daily gain and feed efficiency). Responses of pigs to supplemental SCFA are inevitably interrelated with their endogenous production from dietary fermentable substrates. In the absence of supplementary organic acids, the quantities of SCFA in the small intestine are usually below 40 mmol/L (Clemens et al., 1975), although Jensen (1998) reported that fibre-rich diets may elevate endogenous production of organic acids to 982 mmoL/day The molar ratio of acetate to propionate to butyrate is indicated to be 50:42:8 in the anterior section and 65:18:17 in the posterior section of the small intestine (Williams et al., 1997).
SCFA and Gut Functionality in Pigs
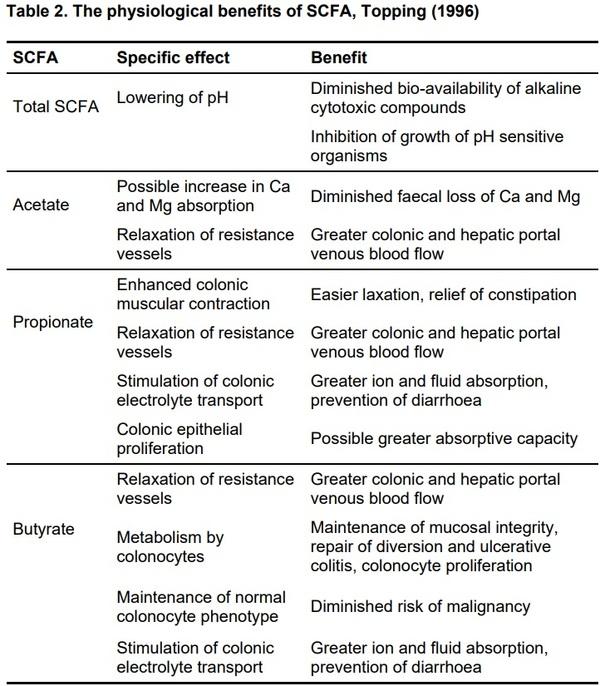
Scarce and sometimes inconsistent information is available in the literature on the role of organic acids in gastrointestinal motility, the development and differentiation of epithelial cells, mucosal immunity and absorptive capacity of the small intestine in pigs. Concerning the role of SCFA on gastric motility and emptying, there is a body of evidence that gastric motor patterns are remote controlled by ileo-colic levels of SCFA, and acetic acid is the most potent to inhibit trans-pyloric digesta flow in pigs (Malbert et al., 1994). As outlined by Buddle & Bolton (1992), the pathophysiology of diarrhoea in pigs is associated with a reduction of surface area due to halved villus height and doubled crypt depth in weaned piglets. Intraluminally produced organic acids can contribute up to 30% of total energy requirements for maintenance in adult pigs, and a part of this energy is also used in the mucosal layer for the synthesis of higher lipids or is catabolised to CO2 (Bugaut, 1987). Galfi & Bokori (1990) observed that 0.17% of Na-butyrate in a diet resulted in substantial increase in the number of cells (33.5%) constituting the microvilli, and in the length of microvilli (30.1%) in the ileum of growing pigs. Whether other dietary acidifiers have similar effects is still unclear. Some studies indicate that organic acids have a stimulatory effect on exocrine pancreatic secretion in pigs (Thaela et al., 1998). A summary of the physiological benefits of SCFA (Topping, 1996) follows in Table 2.
SCFA and Performance of Pigs
There are numerous reports concerning the relationship between supplemental SCFA and pig’s performance. In general, it seems that these acids can nearly compensate for the effects of antibiotic growth promoters (Schöner, 2001), although these effects are less consistent (Partanen and Mroz, 1999).
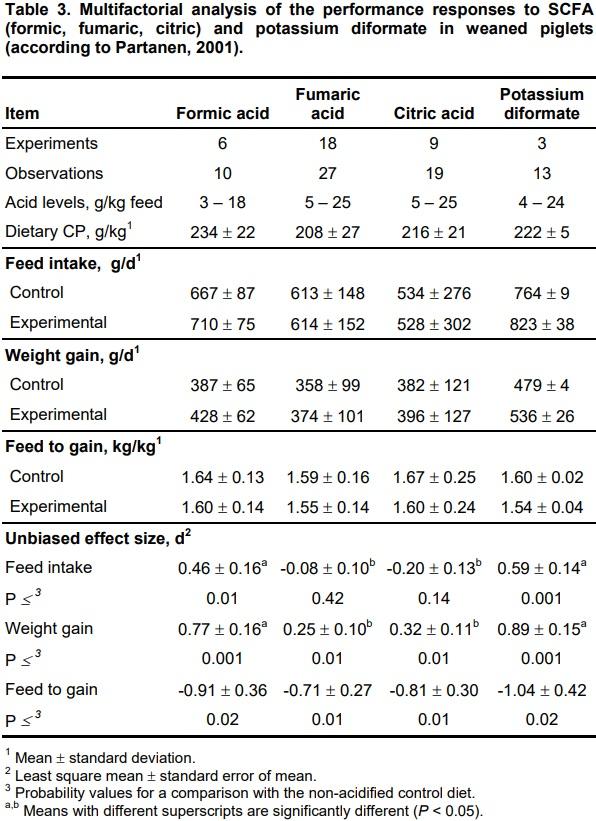
From the data compiled with the multifactorial analysis (Table 3), it appears that the efficacy of some individual organic acids or their salts enhance the growth and feed to gain ratio of weaned and growing-finishing pigs, although there is a relatively large variation in responses due to:
• differences in type and doses of supplemented organic acids;
• differences in type of diets and their acid-base or buffering capacity;
• differences in the level of intraluminal production of SCFA in particular segments of the gastrointestinal tract by inhabiting microflora;
• differences in the quantity of fermentable carbohydrate substrates in the diet for bacterial growth, colonization and activity resulting in SCFA production;
• presence or absence of maternal vaccination against pathogens;
• presence or absence of receptors for bacterial colonization on the epithelial villi;
• weaning age and time after weaning (in the case of piglets);
• existing level of performance;
• hygiene and welfare standards (density per pen, ventilation intensity and area, cleaning frequency etc.).
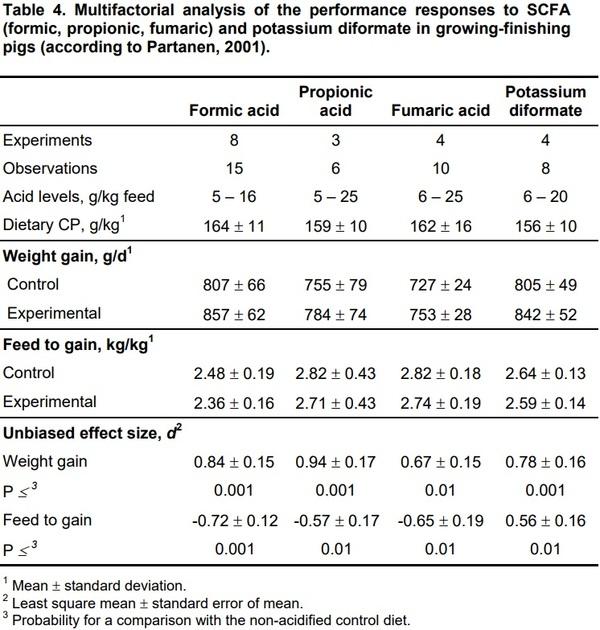
There is some evidence that supplementary SCFA or their salts may be beneficial also for growing-finishing pigs (Table 4).
t can be concluded that the growth response of growing-finishing pigs increased with the dietary crude protein content, whereas the existing level of performance had no effect. The performance response was greater in the growing phase than in the finishing phase. Also, Gauthier (2002) reported consistent positive zootechnical effects in the growing-finishing pigs under practical Canadian conditions.
In sows, organic acids may have anti-agalactic properties and/or prevent bacteriuria of the urinary tract (e.g., benzoic acid). These may further contribute to improved digestion, absorption and retention of organic and inorganic nutrients, and subsequently a reduced ammonia volatilization (“acidic rains”) and specific pollutants (Mroz et al., 1997a,b)
Overall, there does not seem to be a source and level of SCFA for pigs, which meets “one fit all” criteria throughout the world. For optimizing the performance responses to supplemental SCFA in locally available herds of pigs, it seems necessary to gain more knowledge on the dietary acid-base balance, fermentable carbohydrate levels and endogenous production of SCFA from locally available feed resources, and subsequently to adjust their source, amount and physicochemical forms (coated/uncoated, solid/liquid) to be administered via feed or drinking water for a specifically targeted pathogen(s) during particular production cycles (post-weaning, growing-finishing or reproductive).
Conclusions
Supplemental and intraluminally generated SCFA can play a profound role as natural, very selective bactericides/bacteriostatics, precursors/bio-regulators of mucosal growth and systemic energy/acid-base metabolism via direct and/or indirect mechanisms. The morphology and function of the mucosa is malleable through most stages of life; both cell proliferation and apoptosis can be altered at all phases of the life cycle. Their local and systemic mode of action is orchestrated with numerous interactive factors related to diets, health status and neuro-hormonal regulatory processes.
While total SCFA availability seems to be a dominant factor in direct/indirect growth and functioning of the digestive tract, the strongest evidence is that butyric acid contributes to epithelial cell proliferation. Better understanding of nutrient interactions on these effects and of the mechanisms mediating the observations described above are still needed. Not only do these factors have importance for understanding of GI physiology but they are crucial for making practical recommendations.
It seems that more consistent responses of pigs to the supplementary SCFA and their salts can be achieved by optimizing their sources, levels at proper ratios of protected to non-protected forms in diets using a more complex (multifactorial) computerized approach (e.g., a Dutch computer model Nutri-Sure, http://www.demolenaar.nl/artikelen/show). For this purpose, many dietary acid-base specific characteristics (dietary electrolyte balance [Na+K-Cl], dUA [Ca+Mg+Na+K-P-Cl-S], buffering capacity, pH, bicarbonate] could be adjusted to a desired (predicted) mode of action (where physiological parameters determining intestinal health as constituted by gut motility, integrity, immunity, and functionality (including gastric, biliary and pancreatic secretory patterns) could be related to the expected levels of production (including corrosion factors, cost-effectiveness and environmental impacts). The economic feasibility of the use of SCFA can be heavily determined by the farm structure and the restrictions and costs imposed by the regional environmental and health safety policies.
This article was originally published in Advances in Pork Production (2005) Volume 16, pg. 169.










.jpg&w=3840&q=75)