Caecal Microbial Population of Growing Grass Cutters (Thyronoyms Swinderianus) Fed Phyllantus Amarus and Pilogstigma Thonngii Leaf Meal Mixture as Partial Replacement for Soya Bean Meal
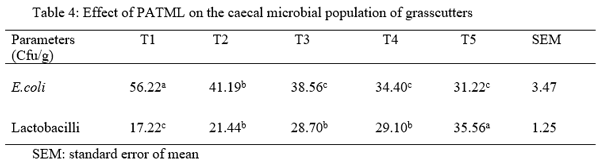
This study was conducted to evaluate the caeca microbial population of growing grass cutters (Thyronoyms swinderianus) fed Phyllantus amarus and Pilogstigma thonngii leaf meal mixture as partial replacement for soya bean meal. A total of thirty five (35) weaned grasscutters of mixed sex between 5-6 weeks with an average weight of 436.1 and 437.0 grams were randomly assigned to five treatment groups in a completely randomized design (CRD). Five experimental diets designated as T1, T2, T3, T4 and T5 were formulated such that soya bean meal was partially replaced by Piliostigma thonningii and Phyllantus amarus leaf mixture (PATML). Feed and water was provided adlibitum throughout the experiment which lasted for 12 weeks. Data obtained was used to determine the caeca microbial population in the animal. Microbial population were influenced by the dietary treatments (P<0.05). Escherichia coli (E. coli) count in the caecum of grass cutters significantly (P<0.05) decreased in T5 compared to that of T1. However, Lactobacilli count significantly (P<0.05) increased in T2, T3, T4 and T5 compared to T1. It could be concluded that partial replacement ofPATLM at 40% repopulates the caecum with beneficial bacteria, which curbs the action of pathogens and controls their population favoring eubiosis and better livestock performance.
Keywords: Phyllantus amarus, Pilogstigma thonngii, grasscutters, pathogens.
- Site of the experiment
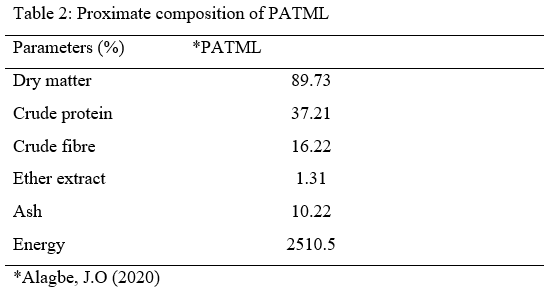
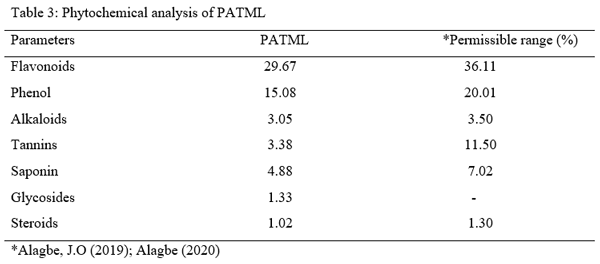
- Collection and processing of test materials
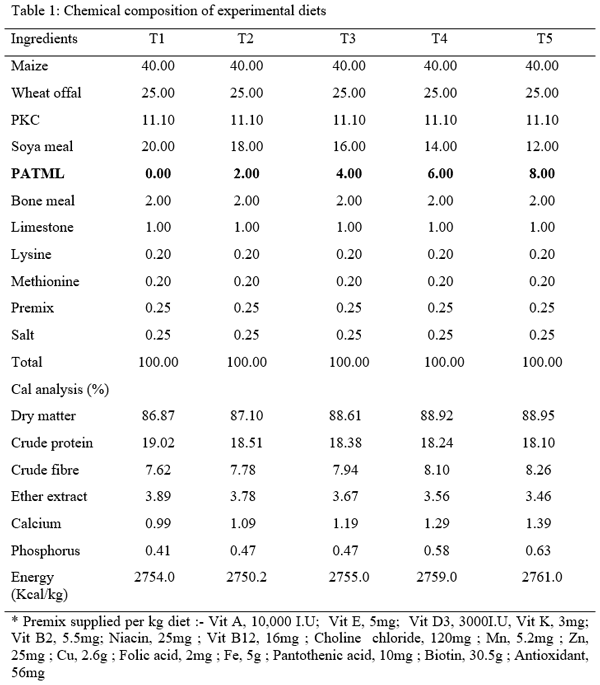
- Formulation of experimental diet
- Pre-experimental operations
- Animal handling and management
- Performance parameters
- Caecal microbial population
- Laboratory analysis
- Statistical analysis
Onwuka, G.I. (2005). Food analysis and instrumentation; theory and practice. African Journal of Biotechnology, 7(1): 1-5.
Boham, B. A and Kocipai, A.C. (1974). Flavonoids and condensed tannins from the leaves of Hawaiian vaccinium vaticulatum and V. calycinium. Journal of Poultry Science. 48 (2):458-463.
Tsado, N.A., Lawal, B., Santali, E.S., Mohammed, A.S., Balarabe, M.M., Ibrahim, H.A and George, J.J. (2015). Phytochemical and acute toxicity profile of aqueous and methanolic extracts of Crateva adansonii leaves in Swiss albino rats. Asian Journal of Biochemistry, 10(4): 173-179.
Olaleye, M.T. (2007). Cytotoxicity and antibacterial activity of methanolic extract of Hibiscus sabdariffa. Journal of Medicinal Plant Research, 1(1): 009-013.
Okwu, D.E and Okwu, M.E. (2004). Chemical composition of Spomdias mombin Linn plant part. Journal of Sustainable Agricultural Development, 6(2): 140 – 147.
Gadde, U., Kim, W.H., Oh, S.T and Lillehoj, H.S. (2017) Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Animal Health Research and Review, 18:26–45.
Liu Y, Song M, Che TM, Bravo D, Pettigrew JE (2012) Anti-inflamma- tory effects of several plant extracts on porcine alveolar macrophages in vitro. Journal of Animal Science, 90:2774–2783.
Krishnan S, Alden N, Lee K (2015) Pathways and functions of gut micro- biota metabolism impacting host physiology. Curr Opin Biotechnol 36:137–145.
Carson CF, Mee BJ, Riley TV (2002) Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron micros- copy. Antimicrob Agents Chemother 46:1914–1920.
Ankri S, Mirelman D (1999) Antimicrobial properties of allicin from garlic. Microbes Infect 1:125–129.
Harbone, I. B (1973) A guide to modern techniques to plant analysis. Chapman and hall, New York, USA 2nd Edition.
A.O.A.C. (2000). Association of Official Analytical Chemists. Official Methods of Analysis 19th Edition Washington, D.C Pages 69-77.
Burt SA, van der Zee R, Koets AP, de Graaff AM, van Knapen F, Gaastra W, Haagsman HP, Veldhuizen EJA (2007) Carvacrol induces heat shock protein 60 and inhibits synthesis of flagellin in Escherichia coli O157: H7. Appl Environ Microbiol 73:4484–4490.
Babajide, S.O., Oluwalana, S.A., Ajala, M.O and Folarin, M.O. (1999). Phytochemical screening of the seeds of Acacia nilotida (Schum and Thonn). The Boprospector, 1(2):27-31.
Faizi, S., Khan, R.A., Mughal, N.R and Malik, M.S., Sajjadi, K.E and Ahmad, A. (2008). Antimicrobial activity of various parts of Polyalthia longifolia: isolation of active principle from the leaves and berries. Phytother Research, 22:907-912.
Galeotti, F.E., Barlie, P., Curir, P., Dolci, M and Lanzotti, V. (2008). Flavonoids from Dianthus caryophyllus and antifungal activity. Journal of Phytochemical Letter, 1: 44-46.
Ighodaro, I., Agunbiade, S.O., Omole, J.O and Kuti, O.A. (2012). Evaluation of the chemical, nutritional, antimicrobial and antioxidant vitamin profiles of Piliostigma thonningii leaves. Research Journal of Medicinal Plant 6(7):537-543.
Alagbe, J.O (2017). Studies on growth performance, nutrient utilization and hematological characteristics of broiler chickens fed different levels of Azolla – Moringa olifera mixture. Greener Journal of Agricultural Sciences, 7(6):145-156.
Peter, S and Bern, S. (2013). The challenge of cost effective poultry and animal nutrition. Optimizing existing and apply novel concepts. Lohmann Inf, 48:38-46.
Chisoro, P. (2015). Potential use of baobab seeds in poultry diets. Animal Feed Manufacturers Association Magazine, 24(2): 52-53.
Fakae, B.B., Cambell, A.M., Barrett, J., Scott, I.M., Teesdale-Spittle, P.H., Liebau, E and Brophy, P.M. (2000). Inhibition of gluthathione transferase from parasitic nematodes by extracts from traditional medicinal plants. Phytother Res, 14(1):630-634.
Lina Sernaite (2017). Plant extracts: antimicrobial and antifungal activity and appliance in plant protection (Review). Lithuanian Journal of Agriculture and Forestry, 3(4):58-66.
Dabofunjo, O.P., Adebayo, A.H., Aliyu, R and Garba, I.H. (2012). The effects of methanolic extract of Philiostigma thonningii leaf on lipid profile of rats. International Journal of Pharmacology, 2(10): 501-508.
Nakayama, N.G., Lindsey, M.L and Michael, L.H. (1993). Inhibition of the infectivity of influenza virus by tea polyphenoids. Antiviral Research 21:289-299.
Oyagade, J.O., Awotoye, O.O., Adewumi, T.J and Thorpe, H.T. (1999). Antibacterial activity of some Nigerian medicinal plants screening. Journal of Biomedical Research 11(3):193-197.
Sofowora, A. (1993). Medicinal plants and traditional medicine. Spectrum Books Ltd, Ibadan, Nigeria, 224-227.
Igoli, J.O., Ogaji, O.G., Tor-Anyin. T.A and Igoli, N.P. (2005). Traditional medicine practice amongst the Igede people of Nigeria part II. African Journal of Traditional Complementary, (2):134-152.
Adeolu, A.A and Sunday, O.O (2013). Anti-inflammatory and analgesics activities of soft drink extract of Phyllantus amarus in some laboratory animals. British Biotechnology Journal, 3: 191-204.
Frankic, T., Volje, M., Salobir, I and Rezar, V. (2009). Use of herbs and spices and their extracts in animal nutrition. Acta Agric. Slov. 94:95-102.
Khartoon, S., Rai, V and Rawat, A. (2004). Comparative pharmacognostic studies of 3 Phyllantus spp. Journal of Ethnopharmacology, 104:79-86.
Gülçin Ì, Sat IG, Beydemir S, Elmastas M, Küfrevioglu ÖI (2004) Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.). Food Chem 87:393–400.
Wang, R., Li, D and Bourne, S. (1998). Can 2000 years of herbal medicine history help us solve problems in the year 2000? Proceedings of Alltech’s 14th Annual Symposium (AAS’98). Kentucky, USA. Pp: 273-291.
Lee, SH., Lillehoj, H.S., Jang S.I., Kim D.K., Ionescu C., Bravo. D. (2010) Effect of dietary curcuma, capsicum, and lentinus on enhancing local immunity against Eimeria acervulina infection. Journal of Poultry Science. 47:89–95
Alagbe, J.O., Olanrewaju, A., Adewemimo, A and Tanimomo, B.K. (2019). Carcass, caeca microbial population and immune parameters of broilers given different levels of mixed lemon grass and garlic extract. Academic Journal of Life Sciences, 5(11):107-111.
Hassan, H.M.A., Amani, W.Y., Eman, F.E., Nafisa, A.E., Eman, R.H and Mohamad, M.A. (2014). Performance, caecum bacteria count and ileum histology of broilers fed different direct fed microbials. Asian Journal of Poultry Science, 8(4):106-114.
Phyo, H.H.K., Kyaw, S.W., Khin, K.L., Kyaw, K.M and Khin, H.S. (2017). Effect of dietary garlic and thyme seed supplementation on the production performance, carcass yield and gut microbial population of broiler chickens. Journal of Scientific Agriculture, 1:269-274.
Yakhkeshi, S., Rahimi, S and Gharid, N.K. (2011). The effects of comparison of herbal extracts, antibiotic, probiotic and organic acid on serum lipids, immune response, GIT microbial population, intestinal morphology and performance of broilers. Journal of Medicinal Plants, 10(37): 80-95.
Duncan, D.B. (1955). Multiple range and multiple F-test. Biometrics 11(1):1-42.
Alagbe, J.O., Sharma,D.O and Xing, Liu. (2019). Effect of Aqueous Piliostigma thonningii leaf extracts on the haematological and serum biochemical indices of broiler chicken. Noble Journal of Agriculture and Food Technology, 1(2): 62-69.
Gary, D and Richard, D.M. (2002). Interrelationship between nutrition and immunity. The institute of Food and Agricultural Sciences Extension Journal, University of Florida. 2-8.
Adeniji, A.A. (2009). Protein and energy requirements of weaner grasscutters. Animal Nutrition and Feed Technology, 9(1):73-79.






.jpg&w=3840&q=75)