The impact of animal products on human health: A 2020 vision of the evidence
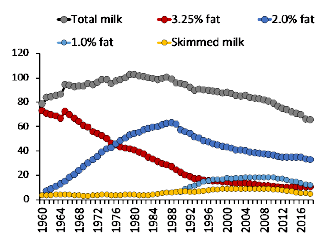
We may be in the midst of a paradigm shift in regard to the role that animal-origin products play in human health. Despite the fact that animal products are amongst the most nutrient-dense foods available to humans, perceptions regarding their effects on health (e.g., increased risks for cardiovascular disease and cancer for dairy and meat, respectively) have become progressively negative. This shift has resulted in important changes in policy and dietary guidelines in several countries, leading to altered patterns of consumption of animal products, and has manifested in trends toward reduced intake of full-fat dairy and beef. Importantly, a substantial body of literature has emerged in recent years that challenges the negative perception linking saturated fats and cardiovascular disease. Furthermore, several recent studies suggest that full-fat dairy consumption may actually reduce the risk of obesity and associated chronic diseases. Similarly, the presumed association between some types of cancer and the consumption of processed or un-processed meat has also been recently put into question. As new studies and more rigorous methods are being used to analyze epidemiological data, we find that strongly held paradigms become weak, as does the degree of confidence on the dietary recommendations to limit these animal products. Further, the constant change in messages received by consumers has led to confusion as to what constitutes healthy dietary habits and may have eroded the confidence in dietary advise arising from nutrition science. Considering the impossibility of using epidemiological research to establish causation, it seems critical to emphasize the importance of randomized control research in order to separate associations from real causal factors. This review has two main objectives: first, to present the historical milestones that have led to the negative shift in consumer’s perceptions towards animal products; second, to summarize current dietary recommendations in light of the best scientific evidence available.
Alberti, K.G.M.M., Eckel, R.H., Grundy, S.M., Zimmet, P.Z., Cleeman, J.I., Donato, K.A., Fruchart, J.C., James, W.P.T., Loria, C.M. and S.C. Smith Jr. 2009. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 120:1640-1645.
Anitschkow N. und S. Chalatow. 1913. Ueber experimentelle Cholester-insteatose und ihre Bedeutung fuer die Entstehung einiger pathologischer Prozesse. Zentrbl. Allg. Pathol. Pathol. Anat. 24:1–9.
Aune, D., Norat, T., Romundstad, P. and L.J. Vatten. 2013. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies–. Am. J. Clin. Nutr 98:1066-1083.
Barrubés, L., N. Babio, N. Becerra-Tomás, N. Rosique-Esteban, and J. Salas-Salvadó. 2019. Association Between Dairy Product Consumption and Colorectal Cancer Risk in Adults: A Systematic Review and Meta-Analysis of Epidemiologic Studies. Advances in Nutrition 10(suppl_2):S190-S211.
Bauman, D. E. and J. M. Griinari. 2001. Regulation and nutritional manipulation of milk fat: low-fat milk syndrome. Livest. Prod. Sci. 70(1-2):15-29.
Baumann, E., P. Chouinard, Y. Lebeuf, D. Rico, and R. Gervais. 2016. Effect of lipid supplementation on milk odd-and branched-chain fatty acids in dairy cows. J. Dairy Sci. 99(8):6311-6323.
Beck, A.L., Heyman, M., Chao, C. and J. Wojcicki. 2017. Full fat milk consumption protects against severe childhood obesity in Latinos. Prev. Med. Rep. 8:1-5.
Belobrajdic, D. P. and McIntosh, G. H. 2000 Dietary butyrate inhibits NMU-induced mammary cancer in rats. Nutrition and Cancer, 36, 217–223.
Bermejo, L. M., B. López-Plaza, C. Santurino, I. Cavero-Redondo, and C. Gómez-Candela. 2019. Milk and dairy product consumption and bladder cancer risk: a systematic review and meta-analysis of observational studies. Advances in Nutrition 10(suppl_2):S224-S238.
Bersaglieri, T., Sabeti, P.C., Patterson, N., Vanderploeg, T., Schaffner, S.F., Drake, J.A., Rhodes, M., Reich, D.E. and J.N. Hirschhorn. 2004. Genetic signatures of strong recent positive selection at the lactase gene. Am. J. Hum. Genet. 74:1111-1120.
Black, R. E., C. G. Victora, S. P. Walker, Z. A. Bhutta, P. Christian, M. de Onis, M. Ezzati, S. Grantham-McGregor, J. Katz, R. Martorell, and R. Uauy. 2013. Maternal and child undernutrition and overweight in low-income and middle-income countries. The Lancet 382(9890):427-451.
Bouvard, V., D. Loomis, K. Z. Guyton, Y. Grosse, F. E. Ghissassi, L. Benbrahim-Tallaa, N. Guha, H. Mattock, K. Straif, and D. Corpet. 2015. Carcinogenicity of consumption of red and processed meat. The Lancet Oncology 16(16):1599-1600.
Calle, E. E. and R. Kaaks. 2004. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature Reviews Cancer 4(8):579-591.
Castelli, W.P. 1988. Cholesterol and lipids in the risk of coronary artery disease--the Framingham Heart Study. Can. J. Cardiol. 4:5A-10A.
Chen, G.C., Szeto, I.M., Chen, L.H., Han, S.F., Li, Y.J., Van Hekezen, R. and L.Q. Qin. 2015. Dairy products consumption and metabolic syndrome in adults: systematic review and metaanalysis of observational studies. Sci. Rep. 5:14606.
Chowdhury, R., Warnakula, S., Kunutsor, S., Crowe, F., Ward, H.A., Johnson, L., Franco, O.H., Butterworth, A.S., Forouhi, N.G., Thompson, S.G. and K.T. Khaw. 2014. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and metaanalysis. Ann. Intern. Med. 160:398-406.
Curry, A., 2013. The milk revolution. Nature 500:20.
Data world bank. 2020. UNICEF, WHO, World Bank: Joint child malnutrition estimates. https://data.worldbank.org/indicator/SH.STA.STNT.ZS?end=2019&start=1983&view=chart&year=1983
Dayton, S. and M. L. Pearce. 1969. Prevention of coronary heart disease and other complications of arteriosclerosis by modified diet. Am J Med 46(5):751-762.
De Heinzelin, J., J. D. Clark, T. White, W. Hart, P. Renne, G. WoldeGabriel, Y. Beyene, and E. Vrba. 1999. Environment and behavior of 2.5-million-year-old Bouri hominids. Science 284(5414):625-629.
de Onis, M. and F. Branca. 2016. Childhood stunting: a global perspective. Maternal & Child Nutrition 12(S1):12-26.
De Souza, R.J., Mente, A., Maroleanu, A., Cozma, A.I., Ha, V., Kishibe, T., Uleryk, E., Budylowski, P., Schünemann, H., Beyene, J. and S.S. Anand. 2015. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. Bmj. 351p.h3978.
Dehghan, M., Mente, A., Rangarajan, S., Sheridan, P., Mohan, V., Iqbal, R., Gupta, R., Lear, S., Wentzel-Viljoen, E., Avezum, A. and P. Lopez-Jaramillo. 2018. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. The Lancet. https://doi.org/10.1016/S0140-6736(18)31812-9
Després, J.-P., B. Lamarche, P. Mauriège, B. Cantin, G. R. Dagenais, S. Moorjani, and P.-J. Lupien. 1996. Hyperinsulinemia as an independent risk factor for ischemic heart disease. New England Journal of Medicine 334(15):952-958. doi: 10.1080/17512433.2018.1519391.
Dong, J.-Y., L. Zhang, K. He, and L.-Q. Qin. 2011. Dairy consumption and risk of breast cancer: a meta-analysis of prospective cohort studies. Breast cancer research and treatment 127(1):23-31.
Elwood, P.C., Givens, D.I., Beswick, A.D., Fehily, A.M., Pickering, J.E. and J. Gallacher. 2008. The survival advantage of milk and dairy consumption: an overview of evidence from cohort studies of vascular diseases, diabetes and cancer. J. Am. Coll. Nutr. 27:723S-734S.
Esposito, K., Chiodini, P., Colao, A., Lenzi, A. and D. Giugliano. 2012. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care 35:2402–2411
Fogelholm, M., N. Kanerva, and S. Männistö. 2015. Association between red and processed meat consumption and chronic diseases: the confounding role of other dietary factors. European journal of clinical nutrition 69(9):1060-1065.
Foote, M. R., S. L. Giesy, G. Bernal-Santos, D. E. Bauman, and Y. R. Boisclair. 2010. t10,c12-CLA decreases adiposity in peripubertal mice without dose-related detrimental effects on mammary development, inflammation status and metabolism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299:R1521-1528.
Forouhi, N.G., Koulman, A., Sharp, S.J., Imamura, F., Kröger, J., Schulze, M.B., Crowe, F.L., Huerta, J.M., Guevara, M., Beulens, J.W. and G.J. van Woudenbergh. 2014. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2:810-818.
García, R. G., M. Y. Rincón, W. D. Arenas, S. Y. Silva, L. M. Reyes, S. L. Ruiz, F. Ramirez, P. A. Camacho, C. Luengas, and J. F. Saaibi. 2011. Hyperinsulinemia is a predictor of new cardiovascular events in Colombian patients with a first myocardial infarction. International journal of cardiology 148(1):85-90.
Gaucheron, F. 2005. The minerals of milk. Reprod. Nutr. Dev. 45: 473-483.
Guyenet, S. J. and M. W. Schwartz. 2012. Regulation of Food Intake, Energy Balance, and Body Fat Mass: Implications for the Pathogenesis and Treatment of Obesity. The Journal of Clinical Endocrinology & Metabolism 97(3):745-755.
Hall, K. D., A. Ayuketah, R. Brychta, H. Cai, T. Cassimatis, K. Y. Chen, S. T. Chung, E. Costa, A. Courville, and V. Darcey. 2019. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell metabolism 30(1):67-77. e63.
Hamley, S. 2017. The effect of replacing saturated fat with mostly n-6 polyunsaturated fat on coronary heart disease: a meta-analysis of randomised controlled trials. Nutr J 16(1):30.
Harcombe, Z., Baker, J.S., Cooper, S.M., Davies, B., Sculthorpe, N., DiNicolantonio, J.J. and f. Grace. 2015. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open heart 2:p.e000196.
Health Canada. 2019. Canada’s dietary guidelines for health profesionals and policy makers. Canada.ca/Foodguide.
Hegsted, D.M., R. B. McGandy, M. L. Myers, and F.J. Stare. 1965. Quantitative effects of dietary fat on serum cholesterol in man. Am. J. Clin. Nutr. 17:281-295.
Heileson, J. L. 2019. Dietary saturated fat and heart disease: a narrative review. Nutrition Reviews.
Holt, P. R. 1999. Dairy foods and prevention of colon cancer: human studies. Journal of the American College of Nutrition 18(sup5):379S-391S.
Holt, P. R., E. O. Atillasoy, J. Gilman, J. Guss, S. F. Moss, H. Newmark, K. Fan, K. Yang, and M. Lipkin. 1998. Modulation of Abnormal Colonic Epithelial Cell Proliferation and Differentiation by Low-Fat Dairy FoodsA Randomized Controlled Trial. JAMA 280(12):1074- 1079.
Huncharek, M., J. Muscat, and B. Kupelnick. 2008. Colorectal cancer risk and dietary intake of calcium, vitamin D, and dairy products: a meta-analysis of 26,335 cases from 60 observational studies. Nutrition and cancer 61(1):47-69.
Jenkins, T. C., R. J. Wallace, P. J. Moate, and E. E. Mosley. 2008. Board-invited review: Recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem. J. Anim. Sci. 86(2):397-412.
Jensen, M.D., Ryan, D.H., Apovian, C.M., Ard, J.D., Comuzzie, A.G., Donato, K.A., Hu, F.B., Hubbard, V.S., Jakicic, J.M., Kushner, R.F. and C.M. Loria. 2014. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J. Am. Coll. Cardiol. 63:2985-3023.
Jensen, R. G. 2002. The composition of bovine milk lipids: January 1995 to December 2000. J. Dairy Sci. 85(2):295-350.
Jeyaraman, M. M., Abou-Setta, A. M., Grant, L., Farshidfar, F., Copstein, L., Lys, J., ... & Zarychanski, R. (2019). Dairy product consumption and development of cancer: an overview of reviews. BMJ open, 9(1), bmjopen-2018.
Johnson, R.K., Appel, L.J., Brands, M., Howard, B.V., Lefevre, M., Lustig, R.H., Sacks, F., Steffen, L.M. and J. Wylie-Rosett. 2009. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation 120:1011-1020.
Johnston, B. C., D. Zeraatkar, M. A. Han, R. W. Vernooij, C. Valli, R. El Dib, C. Marshall, P. J. Stover, S. Fairweather-Taitt, and G. Wójcik. 2019. Unprocessed red meat and processed meat consumption: dietary guideline recommendations from the Nutritional Recommendations (NutriRECS) Consortium. Ann Intern Med 171:756-764.
Kannel, W.B., Castelli, W.P. and Gordon, T., 1979. Cholesterol in the prediction of atherosclerotic disease: new perspectives based on the Framingham Study. Ann. Intern. Med. 90:85-91.
Kannel, W.B., Dawber, T.R., Kagan, A., Revotskie, N. and Stokes, J., 1961. Factors of risk in the development of coronary heart disease—six-year follow-up experience: the Framingham Study. Ann. Intern. Med. 55:33-50.
Kennedy, A., K. Martinez, S. Schmidt, S. Mandrup, K. LaPoint, and M. McIntosh. 2010. Antiobesity mechanisms of action of conjugated linoleic acid. The Journal of nutritional biochemistry 21(3):171-179.
Keys, A., 1953. Atherosclerosis: a problem in newer public health. Atherosclerosis 1:19.
Keys, A., Aravanis, C., Blackburn, H.W., Van Buchem, F.S., Buzina, R., Djordjevic, B.D., Dontas, A.S., Fidanza, F., Karvonen, M.J., Kimura, N. and D. Lekos. 1966. Epidemiological studies related to coronary heart disease: characteristics of men aged 40-59 in seven countries. Acta Med. Scand. Suppl. 460:1–392.
Konstantinov, I.E., Mejevoi, N. and Anichkov, N.M., 2006. Nikolai N. Anichkov and his theory of atherosclerosis. Tex. Heart Inst. J. 33:417.
Krauss, R.M., 2005. Dietary and genetic probes of atherogenic dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 25:2265-2272.
Kruth, H. S. 2001. Lipoprotein cholesterol and atherosclerosis. Curr. Mol. Med. 1:633-653.
Kuzdzal-Savoie, S. 1964. Comparison of branched chain fatty acids in the feed and the milk of cows. Pages 287-294 in Proc. Annales de Biologie Animale, Biochimie, Biophysique.
Lamarche, B., A. Tchernof, S. Moorjani, B. Cantin, G. R. Dagenais, P. J. Lupien, and J.-P. Després. 1997. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men: prospective results from the Qué bec Cardiovascular Study. Circulation 95(1):69-75.
Lefevre, M., Champagne, C.M., Tulley, R.T., Rood, J.C. and M.M. Most. 2005. Individual variability in cardiovascular disease risk factor responses to low-fat and low-saturated-fat diets in men: body mass index, adiposity, and insulin resistance predict changes in LDL cholesterol–.Am. J. Clin. Nut. 82:957-963.
Leren, P. 1970. The Oslo diet-heart study. Eleven-year report. Circulation 42(5):935-942.
Liebert, A., López, S., Jones, B.L., Montalva, N., Gerbault, P., Lau, W., Thomas, M.G., Bradman, N., Maniatis, N. and D.M. Swallow. 2017. World-wide distributions of lactase persistence alleles and the complex effects of recombination and selection. Hum. Genet. 136:1445-1453.
Lock, A. L. and D. E. Bauman. 2004. Modifying milk fat composition of dairy cows to enhance fatty acids beneficial to human health. Lipids 39(12):1197-1206.
López-Plaza, B., L. M. Bermejo, C. Santurino, I. Cavero-Redondo, C. Álvarez-Bueno, and C. Gómez-Candela. 2019. Milk and Dairy Product Consumption and Prostate Cancer Risk and Mortality: An Overview of Systematic Reviews and Meta-analyses. Adv Nutr 10(suppl_2):S212-S223.
Lustig, R. H. 2010. Fructose: Metabolic, hedonic, and societal parallels with ethanol. J. Am. Diet. Assoc.110:1307-1321.
Luz, P. L. D., Favarato, D., Faria-Neto Junior, J. R., Lemos, P., and A.C.P. Chagas. 2008. High ratio of triglycerides to HDL-cholesterol predicts extensive coronary disease. Clinics 63:427-432.
Mao, Q.-Q., Y. Dai, Y.-W. Lin, J. Qin, L.-P. Xie, and X.-Y. Zheng. 2011. Milk Consumption and Bladder Cancer Risk: A Meta-Analysis of Published Epidemiological Studies. Nutrition and Cancer 63(8):1263-1271.
Mensink, R.P., Zock, P.L., Kester, A.D. and M.B. Katan. 2003. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am. J. Clin. Nut. 77:1146-1155.
Micha, R. and D. Mozaffarian. 2008. Trans fatty acids: effects on cardiometabolic health and implications for policy. Prostaglandins Leukot. Essent. Fatty Acids 79:147-152.
Missmer, S. A., S. A. Smith-Warner, D. Spiegelman, S.-S. Yaun, H.-O. Adami, W. L. Beeson, P. A. Van Den Brandt, G. E. Fraser, J. L. Freudenheim, and R. A. Goldbohm. 2002. Meat and dairy food consumption and breast cancer: a pooled analysis of cohort studies. International journal of epidemiology 31(1):78-85.
Monette, J.S., Hutchins, P.M., Ronsein, G.E., Wimberger, J., Irwin, A.D., Tang, C., Sara, J.D., Shao, B., Vaisar, T., Lerman, A. and J.W. Heinecke. 2016. Patients with coronary endothelial dysfunction have impaired cholesterol efflux capacity and reduced HDL particle concentration. Circ. Res. 119:83-90.
Mozaffarian, D., 2014. Saturated fatty acids and type 2 diabetes: more evidence to re-invent dietary guidelines. Lancet Diabetes Endocrinol. 2:770-772.
Mozaffarian, D., Benjamin, E.J., Go, A.S., Arnett, D.K., Blaha, M.J., Cushman, M., Das, S.R., de Ferranti, S., Després, J.P., Fullerton, H.J. and V.J. Howard. 2015. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation CIR-0000000000000350.
Mozaffarian, D., Cao, H., King, I.B., Lemaitre, R.N., Song, X., Siscovick, D.S. and G.S. Hotamisligil. 2010. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in US adults: a cohort study. Ann. Intern. Med. 153:790-799.
MRC. 1968. Controlled trial of soya-bean oil in myocardial infarction. Lancet 2(7570):693-699.
Nelson, G., J. Bogard, K. Lividini, J. Arsenault, M. Riley, T. B. Sulser, D. Mason-D’Croz, B. Power, D. Gustafson, and M. Herrero. 2018. Income growth and climate change effects on global nutrition security to mid-century. Nature Sustainability 1(12):773-781.
Norris, G. H. and C. N. Blesso. 2017. Dietary sphingolipids: Potential for management of dyslipidemia and nonalcoholic fatty liver disease. Nutrition reviews 75(4):274-285.
O’Donnell-Megaro, A.M., Barbano, D.M. and Bauman, D.E., 2011. Survey of the fatty acid composition of retail milk in the United States including regional and seasonal variations. J. Dairy Sci. 94:59-65.
Oomen, C.M., Ocké, M.C., Feskens, E.J., van Erp-Baart, M.A.J., Kok, F.J. and Kromhout, D., 2001. Association between trans fatty acid intake and 10-year risk of coronary heart disease in the Zutphen Elderly Study: a prospective population-based study. The Lancet 357:746-751.
Ordovas, J.M. 2005. Diet-heart hypothesis: will diversity bring reconciliation?. Am J Clin Nutr. 82(5):919-20.
Park, Y.W. ed., 2009. Bioactive components in milk and dairy products. John Wiley & Sons. Parodi, P. 2009. Milk fat nutrition. Pages 28-51. Wiley Online Library.
Parodi, P.W., 2009. Has the association between saturated fatty acids, serum cholesterol and coronary heart disease been over emphasized?. Int. Dairy J. 19:345-361.
Pierre, F., R. Santarelli, S. Taché, F. Guéraud, and D. E. Corpet. 2008. Beef meat promotion of dimethylhydrazine-induced colorectal carcinogenesis biomarkers is suppressed by dietary calcium. British Journal of Nutrition 99(5):1000-1006.
Pimpin, L., Wu, J.H., Haskelberg, H., Del Gobbo, L. and D. Mozaffarian. 2016. Is butter back? A systematic review and meta-analysis of butter consumption and risk of cardiovascular disease, diabetes, and total mortality. PLoS One 11:p.e0158118.
Poortvliet, P. C., S. Bérubé-Parent, V. Drapeau, B. Lamarche, J. E. Blundell, and A. Tremblay. 2007. Effects of a healthy meal course on spontaneous energy intake, satiety and palatability. British Journal of Nutrition 97(3):584-590.
Prentice, A.M., 2014. Dairy products in global public health–. Am. J. Clin. Nutr. 99:1212S- 1216S.
Rachid, M., C. Matar, J. Duarte, and G. Perdigon. 2006. Effect of milk fermented with a Lactobacillus helveticus R389(+) proteolytic strain on the immune system and on the growth of 4T1 breast cancer cells in mice. FEMS Immunology & Medical Microbiology 47(2):242-253.
Ramsden, C.E., Zamora, D., Majchrzak-Hong, S., Faurot, K.R., Broste, S.K., Frantz, R.P., Davis, J.M., Ringel, A., Suchindran, C.M. and J.R. Hibbeln. 2016. Re-evaluation of the traditional dietheart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968-73). Bmj. 353:1246.
Ran-Ressler, R. R., S. Bae, P. Lawrence, D. H. Wang, and J. T. Brenna. 2014. Branched-chain fatty acid content of foods and estimated intake in the USA. British journal of nutrition 112(4):565-572.
Rautiainen, S., Wang, L., Lee, I.M., Manson, J.E., Buring, J.E. and H.D. Sesso. 2016. Dairy consumption in association with weight change and risk of becoming overweight or obese in middle-aged and older women: a prospective cohort study–3. Am. J. Clin. Nutr. 103:979-988.
Ravnskov, U., de Lorgeril, M., Diamond, D.M., Hama, R., Hamazaki, T., Hammarskjöld, B., Hynes, N., Kendrick, M., Langsjoen, P.H., Mascitelli, L. and K.S. McCully. 2018. LDL-C Does Not Cause Cardiovascular Disease: a comprehensive review of current literature. Expert. Rev. Clin. Pharmacol. [Epub ahead of print].
Rice, B.H., Cifelli, C.J., Pikosky, M.A. and G.D. Miller. 2011. Dairy components and risk factors for cardiometabolic syndrome: recent evidence and opportunities for future research. Adv. Nutr. 2:396-407.
Rico, D. E., S. H. Preston, J. M. Risser, and K. J. Harvatine. 2015. Rapid changes in key ruminal microbial populations during the induction of and recovery from diet-induced milk fat depression in dairy cows. The British journal of nutrition 114(3):358-367.
Rohatgi, A., Khera, A., Berry, J.D., Givens, E.G., Ayers, C.R., Wedin, K.E., Neeland, I.J., Yuhanna, I.S., Rader, D.R., de Lemos, J.A. and P.W. Shaul. 2014. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 371:2383-2393.
Sachdeva, A., Cannon, C.P., Deedwania, P.C., LaBresh, K.A., Smith Jr, S.C., Dai, D., Hernandez, A. and G.C. Fonarow. 2009. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am. Heart J. 157:111-117.
Sacks, F. M., A. H. Lichtenstein, J. H. Wu, L. J. Appel, M. A. Creager, P. M. Kris-Etherton, M. Miller, E. B. Rimm, L. L. Rudel, and J. G. Robinson. 2017. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation 136(3):e1-e23.
Saely, C. H., Rein, P. and H. Drexel. 2007. The metabolic syndrome and risk of cardiovascular disease and diabetes: experiences with the new diagnostic criteria from the International Diabetes Federation. Horm. Metab. Res. 39:642–650.
Saliba, L., R. Gervais, Y. Lebeuf, and P. Y. Chouinard. 2014. Effect of feeding linseed oil in diets differing in forage to concentrate ratio: 1. Production performance and milk fat content of biohydrogenation intermediates of α-linolenic acid. Journal of Dairy Research 81(1):82-90.
Siri-Tarino, P.W., Sun, Q., Hu, F.B. and R.M. Krauss. 2010. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 91:535-546.
Smilowitz, J. T., Dillard, C. J., and J.B. German. 2005. Milk beyond essential nutrients: the metabolic food. Aust. J. Dairy Technol. 60:77.
Soedamah-Muthu, S.S., Ding, E.L., Al-Delaimy, W.K., Hu, F.B., Engberink, M.F., Willett, W.C. and J.M. Geleijnse. 2010. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies–. Am. J. Clin. Nutr. 93:158-171.
Statistics Canada. Food available in Canada; Table: 32-10-0054-01. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=3210005401
Stone, N.J., Robinson, J.G., Lichtenstein, A.H., Merz, C.N.B., Blum, C.B., Eckel, R.H., Goldberg, A.C., Gordon, D., Levy, D., Lloyd-Jones, D.M. and P. McBride. 2014. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am Coll. Cardiol. 63:2889-2934.
Thompson, J. C., S. Carvalho, C. W. Marean, and Z. Alemseged. 2019. Origins of the human predatory pattern: The transition to large-animal exploitation by early hominins. Current Anthropology 60(1).
Turpeinen, O., M. J. Karvonen, M. Pekkarinen, M. Miettinen, R. Elosuo, and E. Paavilainen. 1979. Dietary prevention of coronary heart disease: the Finnish Mental Hospital Study. Int J Epidemiol 8(2):99-118.
Ulbricht, T.L.V. and D.A.T, Southgate. 1991. Coronary heart disease: seven dietary factors. The Lancet 338:985-992.
Ulven, S. M., K. B. Holven, A. Gil, and O. D. Rangel-Huerta. 2019. Milk and dairy product consumption and inflammatory biomarkers: an updated systematic review of randomized clinical trials. Advances in Nutrition 10(suppl_2):S239-S250.
USDA/USDHHS. U.S. Department of Agriculture and U.S. Department of Health and Human Services. 2010. Dietary Guidelines for Americans, 2010. 7th Edition, Washington, DC: U.S. Government Printing Office, December 2010. http://www.cnpp.usda.gov/DGAs2010-PolicyDocument.htm
Vesper, H., E.-M. Schmelz, M. N. Nikolova-Karakashian, D. L. Dillehay, D. V. Lynch, and A. H. Merrill Jr. 1999. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. The Journal of nutrition 129(7):1239-1250.
Victora, C. G., M. de Onis, P. C. Hallal, M. Blössner, and R. Shrimpton. 2010. Worldwide Timing of Growth Faltering: Revisiting Implications for Interventions. Pediatrics 125(3):e473-e480.
Villeneuve, M.-P., Y. Lebeuf, R. Gervais, G. Tremblay, J. Vuillemard, J. Fortin, and P. Chouinard. 2013. Milk volatile organic compounds and fatty acid profile in cows fed timothy as hay, pasture, or silage. J. Dairy Sci. 96(11):7181-7194.
Wang, Y., and S. Li. 2008. Worldwide trends in dairy production and consumption and calcium intake: is promoting consumption of dairy products a sustainable solution for inadequate calcium intake?. Food Nutr. Bull. 29:172-185.
Willett, W.C. 2012. Dietary fats and coronary heart disease. J. Intern. Med. 272:13-24.
Windaus A. 1910. Ueber der Gehalt normaler und atheromatoser Aorten an Cholesterol und Cholesterinester. Zeitschrift. Physiol. Chemie. 67:174.
Wongtangtintharn, S., H. Oku, H. Iwasaki, and T. Toda. 2004. Effect of branched-chain fatty acids on fatty acid biosynthesis of human breast cancer cells. Journal of Nutritional Science and Vitaminology 50(2):137-143.
Wu, S. H., Liu, Z. and S.C. Ho. Metabolic syndrome and all-cause mortality: a meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 25:375–384.
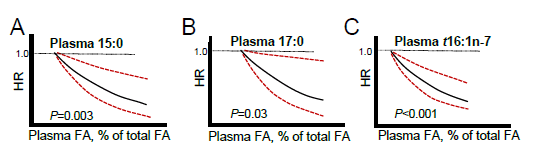
Yakoob, M.Y., Shi, P., Hu, F.B., Campos, H., Rexrode, K.M., Orav, E.J., Willett, W.C. and D. Mozaffarian. 2014. Circulating biomarkers of dairy fat and risk of incident stroke in US men and women in 2 large prospective cohorts–. Am. J. Clin. Nutr. 100: 1437-1447.
Yanagi, S., Yamashita, M. and Imai, S. (1993) Sodium butyrate inhibits the enhancing effect of high fat diet on mammary tumorigenesis. Oncology:50, 201–204.
Yang, Q., Zhang, Z., Gregg, E.W., Flanders, W.D., Merritt, R. and F.B. Hu. 2014. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern. Med. 174:516-524.
Yang, Z., S. Liu, X. Chen, H. Chen, M. Huang, and J. Zheng. 2000. Induction of apoptotic cell death and in vivo growth inhibition of human cancer cells by a saturated branched-chain fatty acid, 13-methyltetradecanoic acid. Cancer research 60(3):505-509.
Yerushalmy, J. and H.E. Hilleboe. 1957. Fat in the diet and mortality from heart disease; a methodologic note. N. Y. State J Med. 57:2343.
Young, S.S. and A. Karr. 2011. Deming, data and observational studies. Significance, 8:116-120.
Zang, J., M. Shen, S. Du, T. Chen, and S. Zou. 2015. The association between dairy intake and breast cancer in western and Asian populations: a systematic review and meta-analysis. Journal of breast cancer 18(4):313-322.







.jpg&w=3840&q=75)