A cross sectional study was undertaken to determine the prevalence and intensity of parasitic infections in dairy cattle in the high tropics of Colombia. A total of 1003 rectal samples were collected from dairy cows at 29 farms between May and June 2014 to represent the number of farms, age groups, and size of the 65,000-cow population in the municipality of San Pedro de los Milagros. Coprological techniques were used to detect gastrointestinal nematodes, liver flukes, coccidian oocysts, and first larval stage counts of Dictyocaulus viviparus. In order of decreasing prevalence, the following parasites were detected: coccidial oocyst (36.7%; 95% CIs, 31.6–42.7), strongyle nematodes (31.6%, 27.8–35.4), liver flukes (30.9%, 21.5–37.5), cestodes (8.4%, 7.1–9.7), and D. viviparus (5.4%, 3.4–7.5). Co-infections by all possible combinations of the three most predominant groups occurred in 11 to 15% of the animals. There were significant differences in infection rates between age groups, with higher risk of liver fluke infection in animals older than 1 year of age (odds ratio (OR) = 3.2), but lower presence for coccidia and strongyles (OR = 0.19 and 0.51, respectively). For Fasciola hepatica, within-herd prevalences of N25% in 16 farms and 94 of 281 (33.5%) animals with N5 eggs per gram (epg) indicate that significant production losses are likely occurring. The variation in the prevalence of gastrointestinal parasites and liver flukes, together with the level of infection among age groups, could be used in integrated management programs to establish selective anthelmintic treatments and select for heritable traits of host resistance. These results serve as a baseline for future studies to determine the success of control measures and should increase awareness that subclinical parasitism is widespread in the livestock sector.
© 2016 The Authors. Published by Elsevier Ltd on behalf of World Federation of Parasitologists. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Epidemiological studies are necessary to determine the potential impact of parasitic infections in particular areas. Antioquia contains 11.7% of Colombia's cattle, or about 2.6 million individuals (Nacional and Agropecuario, 2016). The high plains of Northern Antioquia contain the largest population of dairy cattle in Colombia with about 310,000 milking cows. The area has a suitable climate for the development of most species of helminth parasites due to abundant grass pasture throughout the year, no dry season, and moderate temperatures. A recent study that monitored the annual weather patterns showed that 24-h temperatures fluctuated between 5 and 20 °C, there were no freezing events, and relative humidity ranged between 50 and 90% (Echeverri et al., 2015). With the above conditions, parasites are likely influencing the productive performance of the herds depending on the intensity of infection and factors like age and nutritional status of the animals.
An anecdotal report from a large dairy farm in the high tropics of Antioquia aimed to determine the frequency of paramphistomids in snails and cattle showed a prevalence for Fasciola hepatica of 80% (51/71 cows) and that two species of snails, Lymnaea truncatula and L. columella, were infected with intra-mollusk stages of the trematodes (Lopez et al., 2008). A more recent study that randomly selected 180 culled bovines sent to a local slaughter in the same area revealed the presence of adult forms of F. hepatica in 41 livers (Correa et al., 2016). According to recent studies in European countries, fasciolosis is undoubtly a major production disease which for the last decade has either increased, or remains high, in spite of control efforts (Charlier et al., 2014). With regard to bovine gastrointestinal nematodes, the epidemiological situation in the country is also largely unknown. A study conducted in 36 farms of Cundinamarca determined the anthelmintic resistance by doing fecal egg count reduction tests (FECRT) and also identified the different types of nematodes (Marquez et al., 2008). In order of decreasing prevalence the following parasites were identified: Cooperia spp. (67%), Haemonchus spp. (13%), Ostertagia spp. (11%), Trichostrongyles spp. (8%). In addition, resistance to albendazol and ivermectin was detected in 17% and 8% of the farms, respectively. Limited and past investigations on Dictiocaulus viviparus in three dairy farms in the high tropics of Antioquia showed a prevalence of 38.6% (49/127) in animals of less than 1 year old (Lopera, 1991). Interestingly, a clear tendency to lower prevalence with increasing age of the calves was also observed. Recent field investigations in mortality cases of 6–8 month calves have shown D. viviparus to be the culprit (personal observations).
In view of the lack of epidemiological information on the prevalence of parasitic infections in Colombia, a cross-sectional coprological study was designed to provide an overview of the situation in the largest dairy area of Antioquia (Fig. 1).
2. Materials and methods
2.1. Study area and animals
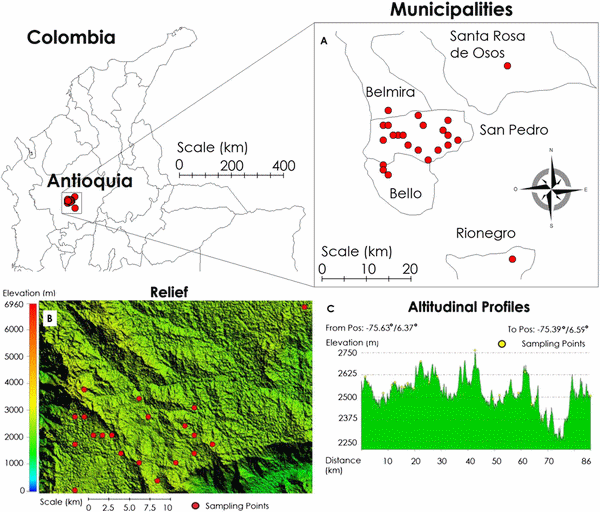
The study was performed at 29 dairy farms between May and July 2014. The farms were located in the municipality of San Pedro de los Milagros, which has a total population of 65,000 cattle. The farms were located in the high tropics of Antioquia at an altitude of 2500 m above sea level, latitude of 6°27′34″N, and longitude of 75°33′28″W (Fig. 2).
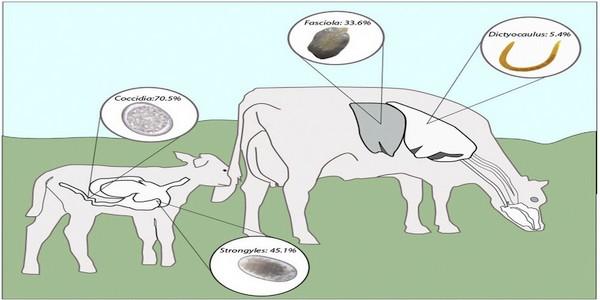
Fig. 1. Prevalence of main internal parasites in young (b1 year) and adult (N1 year) dairy cows in the high tropics of Antioquia, Colombia.
Fig. 2. (A) Locations of the study farms within the Antioquian and Colombia maps. (B) and (C) Landscape of the area, notice the altitude is between 2490 and 2750 m above sea level.
N3 years (n= 548). The sample size for each farm, number of farms, and ages within each farm, were chosen to be proportional to the municipality's population of 65,000 head,with an expected prevalence of 50%,margin of error of 3.1% (≤5%), and a 95% confidence interval. The breeds of the cows were Holstein, Holstein-Jersey, Jersey and others. The number of farms with land area of b50, 50–100, and N100 “fanegas” (1 fanega=0.66 ha) were 19, 7 and 1, respectively. There was no data available for two herds. The management system practiced in the area is primarily an intensive rotational grazing systemon Kikuyu (Penisetum clandestinum)monoculture pastures with no confinement of adult cows. Calves are usually kept in separate paddocks between the ages of 3–9 months. They are then placed with replacement heifers until they are ≥15 months old. Pastures are rested for approximately 30–40 days, and occupation days vary with grazing density of animals. The animals received anthelmintic treatment according to the criteria of the farmer; Table A shows the type of treatments and frequency of administration when the information was provided.
2.2. Sample collection and examination
Fecal samples were collected directly from the rectum and shipped within 12 h to the laboratory for processing. When possible, samples were separated and subjected to three techniques: modified 3-chambered McMaster method for Strongylidae fecal egg counts (FEC) and coccidian oocysts (Vadlejch et al., 2011), Baermann technique for D. viviparus larvae detection (Rode and Jorgensen, 1989), and a sedimentation technique for F. hepatica egg counts. Dilutions were made to estimate the exact number of coccidian oocysts.
Table A. Number of farms grouped according to the antiparasitic drugs used and the frequency of administration (n = 29a).
For the sedimentation technique, 2 g of feces from one sample was added to 25 mL of soapy water in a Falcon tube. The contents were mixed thoroughly using a glass rod and were poured through a tea strainer to remove large debris. The solution was further passed through a sieve (mesh aperture 210 mm) into a conical flask and water was run through the sieve to ensure no eggs remained attached to the sieve. The filtrate was then allowed to sediment for 10 min after which time the supernatant was siphoned off, taking care not to disturb the precipitated matter. The sediment was stained with two drops of methylene blue, and the entire sediment was placed on a glass slide and viewed under a dissecting microscope (Olympus CH30RF100). F. hepatica eggs were counted and this number was used to calculate the number of eggs per gram (epg).
Although there is no consensus to define the intensity of infection, levels of strongyle infections were classified as low (b200 epg), moderate (201–700 epg) and high (N700 epg) to allow comparisons with similar studies (Pfukenyi et al., 2007) and to establish therapeutic thresholds (Malone and Craig, 1990). Similarly, coccidian oocyst levels were arbitrarily divided into: negative (zero), low (1–1000 oocyst per gram (opg)), moderate (1000–5000 opg), and high (N5000 opg); however, it must be emphasized that no specific excretion rates for coccidia have been defined as relevant from a clinical or economic standpoint.
2.3. Statistical analysis
Data was entered into Excel worksheets and exported to Stata 12.0 (StataCorp, 2011) for analysis. Descriptive statistics were computed for most variables. A univariate logistic regression model was constructed, and the relationship between the prevalence of infections and independent variables was analyzed using coprological status (positive/negative) as the dependent variable. The variables introduced in the model included: age, total number of animals on the farm, breed, and deworming practices (yes/no). If deworming had been applied within the last month, it was included as a positive response. The results are presented as odds ratios (OR) along with their 95% confidence intervals.
3. Results
A descriptive analysis with the overall prevalence of the different parasite groups detected is summarized in Table B. Parasite infections by coccidian spp., strongyle spp., and F. hepatica had the highest levels of prevalence with 36.7, 31.6 and 29.5%, respectively. Less prevalent were Moniezia spp. (8.4%), D. viviparus (5.4%), and other parasites detected (2.3%) that included Trichuris, Toxocara and Strongyloides. Concurrent infections occurred in 144/927 (15.5%) for coccidian and strongyles, 96/910 (10.5%) for coccidian and liver fluke, and 105/910 (11.5%) for liver fluke and strongyles. There were 43/927 (4.6%) with coinfection to coccidian, strongyles and F. hepatica.
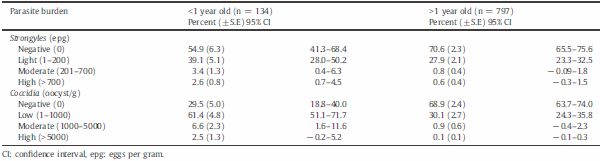
There were important differences, not only in prevalence of infections among age groups, but also in egg counts (Table C). For strongyle eggs, calves (b1 year old) had fewer negative animals than older groups (54.9 vs 70.4%), as well as a higher percentage of animals with light, moderate, and heavy infections (45.1 vs 29.6%). Similarly, the percentage of animals without coccidian oocysts was lower in calves than older animals, with values of 29.5 and 68.9%, respectively. There were 2.5% of calves with high excretion of coccidian oocysts, whereas only 0.1% of the adults were high shedders. F. hepatica was detected in 282 animals, of which only 13 were below 1-year old. Of those that were positive, 37.9% (107/282) had FEC N 5 epg. The age distribution of those with FEC N 5 epg was b1 year (n = 6), 1–b2 years (n = 12), 2–b3 years (n = 27) and = or N3 years (n = 62).
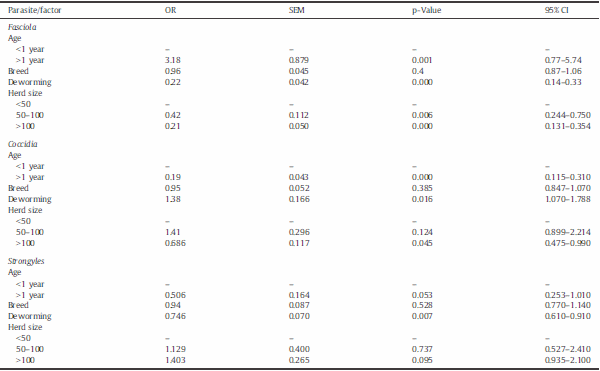
The univariate analysis of risk factors showed statistically significant associations for age, herd size, and deworming, and no significant influence for cattle breed (Table D). An increased OR of 3.2 was shown for adults to have liver flukes, whereas, for coccidia and strongyles, the odds were 0.2 and 0.5, respectively. Deworming with fenbendazole and ivermectin had some protective effect against strongyles (OR = 0.7, p b 0.01) and, paradoxically, for liver flukes (OR = 0.2, p b 0.01). Larger herd sizes showed lower levels of liver fluke infection than small-scale farms, with an OR of 0.4 and 0.2 for herds of 50–100 and N100 animals, respectively. For coccidia, lower levels of infection occurred only in the larger herds (OR = 0.7, p b 0.05). No effect of herd size was observed for strongyle nematodes.
Table B. Prevalence (%) of gastrointestinal parasites, liver flukes, and lungworm in dairy cattle in the high tropics of Antioquia, Colombia.
Table C. Number of cattle by age with different levels of strongyles and coccidian infections in the high tropics of Antioquia, Colombia.
4. Discussion
The purpose of this study was to document the prevalence of internal parasites in cattle of a major Colombian dairy area. Similar prevalences of about 30% were observed for coccidia, strongyles, and F. hepatica; double co-infections by all possible combinations of these groups occurred in 11 to 15% of the animals, and 4.6% had all three parasites genera. Another interesting finding was the higher prevalence and intensity of infection by coccidia and strongyles in younger animals, but not for F. hepatica. Calves on pasture generally harbor greater burdens of coccidia and strongyle infections than adults (Pfukenyi et al., 2007; Lima, 1998), presumably because they are immunologically naïve and less capable of preventing parasite establishment. By contrast, F. hepatica was most prevalent in adults, probably reflecting an age-specific pattern of exposure as well as its long life cycle which requires 2–3 months for the development of larvae into mature worms. It would have been of interest to further separate these age groups and the adults according to their physiological state. It is known that parasite intensity rises in the periparturient period; this is thought to occur from prioritization of reproduction over anti-parasite immunity (Beasley et al., 2010; Viana et al., 2009). Also, the stages from birth to early adulthood should ideally be separated to reflect the particular management system of the farm, as this was shown to be quite different among producers. Although most animals will stimulate a protective immunity against many species of nematodes after several months of exposure on pasture, there are certain parasites such as Ostertagia, for which an immune response is not so evident until at or after 2 years of age (Gasbarre et al., 2001). Obviously, knowing the nematode genera is an important consideration that cannot be overlooked when interpreting fecal egg counts, as for Ostertagia cattle between the ages of 1 and 2 years could possibly be grouped with the younger calves. In this regard, this study was limited in scope because it did not characterize the genera of nematodes present in the animals.
Table D. Univariate analysis of risk factors associated with Fasciola hepatica and gastrointestinal parasite infections in dairy cattle. Results are presented as odd ratios (OR), standard error of the mean (SEM), and 95% confidence intervals (CI).
Many factors that may influence exposure and parasite establishment must be considered when evaluating how parasites are distributed among age groups. For the purposes of finding a control strategy and selecting a resistance trait, it may be useful to record the number of animals shedding different levels of eggs. In this respect, the prevalence of strongyle negative animals and those shedding different levels of eggs were very similar to that reported for a communal grazing system (Pfukenyi et al., 2007). In the present study, overall prevalence in calves and adults was 45% and 29.4%, respectively. In addition, the majority of calves had low strongyle egg outputs (b200 epg), and only a few were heavy shedders (N700 epg), which is similar to the trend that was observed in their communal grazing system (Pfukenyi et al., 2007). From a selective breeding standpoint, it has been shown that the number of nematode eggs per gram in feces of pasture cattle has a heritability trait of approximately 0.30, which offers great potential to genetically control resistance/susceptibility to parasites (Gasbarre et al., 2001).
The spectrum of strongyles that was represented here was not studied. However, in a study aimed to detect the degree of resistance against anthelmintics in 36 farms of other Colombian municipalities of the high tropics, morphological differentiation by coproculture showed that Cooperia and Haemonchus accounted for 67% and 13% of the Strongylidae species (Marquez et al., 2008). These are also the two nematode species that are more prevalent in dairy farms reported in other tropical countries (Lima, 1998; Jimenez et al., 2007; Vazquez et al., 2004).
From a practical viewpoint of parasite control, this study showed some relevant information. Generally, producers use anthelmintics in anticipation of better animal performance and thus an increase in profits. However, this practice has resulted in misguided overuse of most antiparasitics to the point that resistance to most classes of drugs is now widespread. This study shows that the vast majority of animals were negative and/or had low parasite burdens, particularly in the older age groups (except for F. hepatica). This is consistent with statements in many technical bulletins for veterinarians and producer advisors that parasites have an aggregated distribution within a herd, and only a small percentage (∼20%) of animals carry the bulk (∼80%) of the parasite population. In this respect, and in light of the emerging anthelmintic resistance, restricting the use of antiparasitics to a few susceptible individuals is a practice that should be advocated at every opportunity as part of a more sustainable means of parasite control. It is interesting to note in this study that deworming had a protective effect against strongyles and F. hepatica; however, the information provided by many farmers was dubious or misleading, as they frequently misunderstood treatment for non-parasitic conditions with use of a particular dewormer, or considered antimicrobial treatment as a dewormer. Lack of veterinary oversight on the use of veterinary drugs is common in rural areas of the country and requires more careful monitoring. As can be seen in Table A the farmers were arbitrarily using ivermectin, fenbendazole or the two combined. The frequency of application did not follow technical criteria such as parasite load, economic losses or clinical signs (i.e., body condition, rough hair coat), and are usually made at regular intervals or coinciding with vaccination programs for foot and mouth disease or serological checks for Brucella.
Whenever possible, a threshold for production-based treatment should be established for gastrointestinal nematodes, lungworm, and liver fluke infections. Although FECs are poor indicators of the level of nematode infection (as compared to serum pepsinogen for subclinical ostertagiosis), they can be used to predict pasture contamination, as they tend to peak around 2 months after turnout (Vercruysse and Claerebout, 2001). When the geometric mean of at least 20 calves after 2 months of turnout was ≥200 epg, the number of calves that would later develop parasitic gastroenteritis was much higher than when it was ≤200 epg (Shaw et al., 1998a; Shaw et al., 1998b), indicating that such a threshold could be set to establish control measures. In the present study, only 6% of calves had FEC ≥ 200 epg, suggesting very low burdens in most farms that would not warrant therapeutic intervention. Again, if Ostertagia had been the main nematode, mean pepsinogen levels were shown to better identify the calf groups with low exposure levels (insufficient to acquire immunity), medium levels (not expected to have negative impacts), or too high (causing reduced weight gains) to warrant control measures (Vercruysse and Claerebout, 2001; Charlier et al., 2010).
With regard to F. hepatica, the production threshold for treatment proposed by several authors has been based on: within-herd prevalence of 25% or greater, fecal egg counts of five epg or higher, and gamma glutamyl transferase levels above 150 units/L (Vercruysse and Claerebout, 2001; Malone and Craig, 1990). In this study, a high within-herd prevalence N25% in 16 farms, and 89 of 269 adult animals with FEC N 5 epg, suggests that a large economic impact is probably occurring through subclinical impairment of feed efficiency, growth, fertility, and loss of milk production (Malone and Craig, 1990; Kaplan, 2001). Unfortunately, estimation of the actual infection level through fecal egg counts is low, which still makes guides for interpreting burdens somewhat of an estimation. More recently, methods based on measuring anti-F. hepatica antibodies in bulk-tank milk have been used to identify herds likely to suffer production losses due to fasciolosis (Charlier et al., 2007). In future studies, these more sophisticated methods could be used to increase awareness of economic impact that fasciolosis is likely having in Antioquian cattle herds. The area studied contains many hills which encourages water collection in the lower parts of the farms. In future studies, the roles of climate, drainage on lower parts of the farms, and, most importantly, pasture management measures should be included to improve the assessment of risk factors for fasciolosis.
Coccidia were detected at different rates of excretion in the majority of fecal samples from calves (∼70%); by contrast, most adults (70%) were negative and those that were positive (30%) were low shedders. In accordance with numerous other studies (Pfukenyi et al., 2007; Bangoura et al., 2011; Lucas et al., 2014), these findings confirm that calves less than one year of age have a high prevalence of coccidia infections and can shed high numbers of oocysts. Production losses associated with subclinical coccidia infection have not been estimated, but are thought to be substantial (Daugschies and Najdrowski, 2005). Although the occurrence of diarrhea was not evaluated in this study, larger scale epidemiological reports have observed a strong correlation between levels of pathogenic Eimeria spp. and incidence of diarrhea in calves (Bangoura et al., 2011). Future studies will also need to identify the species of Eimeria, as most are not considered to be pathogenic.
In conclusion, this study provides baseline data on the prevalence of gastrointestinal parasites that can be used for comparison with future studies. The results suggest that a substantial economic impact is occurring on the livestock sector, particularly with fasciolosis. In addition to diagnosing parasite burdens based on FEC, more refined tools such as bulk tank milk analysis for anti- F. hepatica antibodies and serum pepsinogen for Ostertagia would help farmers to determine the likely impact of parasites on production.
Acknowledgements
This work was supported by Vecol, Zoolab and the University of Antioquia, School of Veterinary Medicine. The authors thank University of Antioquia and the Centauro Sustainability Project 2013–2014; we also thank Carlos Ortega for the graphic design.
This article was originally published in Parasite Epidemiology and Control 1 (2016) 124–130. http://dx.doi.org/10.1016/j.parepi.2016.05.001. This is an Open Access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).









.jpg&w=3840&q=75)