Introduction
Traditionally, ensiling was used to preserve forage material for feeding animals after the cropping seasons. Recently, this method has a potential to store the large quantities of biomass feed stocks to produce energy through lignocellulosic ethanol production (Sipos et al., 2009). Similarly, there is a growing interest in sweet sorghum (Sorghum bicolor) in recent years because it has the potential to be used as bioenergy crop for lignocellulosic ethanol production. This crop is resistance to drought, saline and disease. In addition, it requires low fertilizer and it is an attractive forage crop for many tropical and subtropical areas including Iran. The high sugar content in sweet sorghum also makes to ensiled for dry season feeding. Therefore, there is an growing interest to study the possibility and convenience of partial or even total substitution of corn silage with sweet sorghum (Colombo et al., 2007). On completion of fermentation, pH, sugar and organic acid content should reasonably remain stable. Hydrolysis of hemicellulose due to the acidic condition is common during fermentation (McDonald et al., 1991). Fermentation by lactic acid bacteria is usually completes within three weeks (Jaster, 1995). However, Ward and de Ondarza (2008) suggested that, corn silage requires at least four months for a full fermentation process. Kleinschmit and Kung (2006) reported that a satisfactory fermentation of corn silage in mini silos requires 361 days of ensiling. In their investigation, the major increase in acetic acid in untreated corn silage occurred between 282 and 361 days, this evidence suggesting that the change in concentration in acetic acid was most likely due to Lactobacillus buchneri. This organism is relatively acid tolerant and can survive for long periods of time in fermented silage (Schmidt et al., 2009). Production of butyric acid by clostridia causes an increase in pH and significant losses in dry matter and energy content that can reach 50% and 20%, respectively (Bolsen, 1995). Studies have shown that aerobic microbial fermentation and the lactic acid bacteria (LAB) activities on plant cell respiration completes within the first month of ensiling (Gary, 1992). Caswell et al. (1983) ensiled sweet sorghum with moisture concentration of 740 g/kg for a period of 31 days and noted that WSC concentration was reduced by 57.5%. Similarly, Linden et al. (1987) ensiled compressed sweet sorghum containing moisture concentration of 660 g/kg and after 155 days of ensiling, 65% of the initial fermentable carbohydrate concentration was preserved. Stokes and Chen (1994) reported, after 56 days of fermentation, ADF, cellulose and CP in corn silage increased and NDF, hemicellulose, and WSC became lower than the original forage. Yahaya et al. (2002) showed that prolonging ensilaging time of high moisture orchard grass would result to excessive loss of DM, WSC, hemicellulose and cellulose in the silages. Calabrò et al. (2007) observed that the ensiling caused a reduction in NEl, OMD, but an increase in structural carbohydrates contents in sorghum. Calabrò et al. (2007) showed that loss of soluble fractions during ensiling was higher for corn than sorghum. Hoffman et al. (2011) investigated the effect of ensiling time on starch-protein matrix in high-moisture corn and they reported that the NH3-N and buffer soluble CP (SCP) concentration increased from 0 to 240 days. It was also been reported that rumen degradability of corn silage (Newbold et al., 2006) and high-moisture corn (Benton et al., 2005) increased with ensiling time. In addition, Philipp et al. (2007) noted reduction in WSC concentration for different varieties of sorghum over 21 days of incubation period.
Despite the importance of sweet sorghum as a bioenergy and forage sources crop, little research has been done on its utilization as an alternative feed resource during critical feed shortage especially in the dry season. Similarly, measurement of undesirable breakdown of nutrients such as WSC and CP during ensiling, which depends on many factors, is not fully understood for sweet sorghum. Furthermore, there is scanty information on the effect of ensiling time on the degradation of structural carbohydrates and alteration of in vitro digenstability (IVD) or gas production (GP) parameters and nutrients composition and lost during ensiling in sweet sorghum. The objective of this experiment was, therefore, to determine the effects of ensiling time on the chemical composition, nutritional characteristics and nutrients lost during ensiling in sweet sorghum forage after 30, 60, 90 and 120 days of ensiling in mini-silos.
Materials and Methods
Study location and crop management
The experiment was carried out at Isfahan University Research Station (31º, 31'N, 5º, 51' E, altitude 1550 m). Sweet sorghum was planted on 25 May, 2012 and harvested after about 120 days with a mean DM concentration of 295 g/kg fresh weight. Potassium sulfate (100 kg/ha) and ammonium phosphate (300 kg/ha) were used in the beginning of the cultivation. After 30 days of growing, 100 kg/ha urea were added to the field. Whole plant including stems, seeds and leaves were collected and chopped to about 2-3 cm in length using a mechanical forage cutter and divided into three equal parts by weight serving as experimental replicates.
Ensiling procedure
Forage pieces were ensiled in PVC containers with 4.0±0.2 kg capacity (cylindrical shape with 50 cm height × 16 cm diameter). Ensiling was done about 20 hours after harvesting so a slight wilting happened from harvest to ensiling time. A tap and hose were attached to the bottom of the silos to drain the effluent. After filling the silos with plant material, they were pressed using a pressing apparatus to expel the air completely. The silos were made air-tight by closing the lid tightly and the lids were lubricated with oil to seal effectively. The laboratory silos (three replicates for each treatment) were placed in a dark room at the average temperature of 18°C until their opening at 30, 60, 90 and 120 days after the storage.
Sampling and chemical analysis
Fresh and ensiled forage
The silages were evaluated after 30, 60, 90 and 120 days of ensiling. Before evaluation, from both sides of silos, about 5 cm of materials were discarded to ensure uniformity of samples. Then, the remaining material was mixed thoroughly. Approximately 2 kg of ensiled material was transferred into a vacuum plastic bag and preserved at -20°C for further experiment. Fresh forage and samples ensiled for 120 days were likewise frozen for 21- d to ensure protocol continuity (Hoffman et al., 2011).
Preparing silage extract
About 30-g sampled fresh silages was mixed with 270 ml distilled water and blended using a kitchen blender for 50 to 60 seconds. The extract was then filtered using four layers of cheesecloth. The pH was determined using a digital pH meter (Metrohm 744, Switzerland). Moreover, the extract was stored at -20°C for analyzing lactate, acetate, butyrate, propionate, ethanol and NH3-N.
Chemical analysis
After being frozen for 21 days, all samples were allowed to thaw and were air dried for 48 hours in a forced-air oven at 55°C. Fine particles of silages were collected after sieving through a 1mm siever and were used to determine chemical composition. Effluent was estimated by volume gain (ml) of exhaust calibratedcollector tube connected via vessel to minisilos. The ether extract (EE), CP and ash were measured according to AOAC (1999) while WSC was measured by phenolsulfuric acid method (Masuko et al. 2005). The UV absorption was recorded at 470 nm wavelength using a spectrophotometer (Jasco V-570 UV/Vis/NIR spectrophotometer, Japan).
Acetate, butyrate, propionate and ethanol were measured by gas chromatography (Crompak, Model CP 9002, The Netherlands) as described by Playne (1985). The lactic acid was estimated by high-performance liquid chromatography (HPLC) method developed by Megias et al. (1993). Ammonia nitrogen was measured using Kjeldahl method (Kjeltec Auto 1030 Analyzer, Sweden) in 50 ml of fresh silage extracts (without digestion) filtered through Whatman filter paper #1 (Filya, 2003). aNDF (using heat-resistant alpha-amylase and corrected for ash) and ADF were measured according to Van Soest et al. (1991) and ADL was measured by hydrolysis method using 72% sulfuric acid (Van Soest and Wine, 1968). The non fibre carbohydrates (NFC) were calculated using the following formula (Ishler and Varga, 2001): NFC = 1000 - [ash + EE + CP + aNDF - NDIP]
Determination of the protein fractions
Borate-phosphate buffer (pH 6.7-6.8) and sodium azide 10% solution (freshly prepared) were used to measure SCP as described by Licitra et al. (1996). The NDIP and acid-detergent insoluble protein (ADIP or C fraction of CNCPS) were estimated according to Van Soest (1973).
Determination of in vitro digestibility
In order to determine in vitro digestibility of DM (IVD), 0.5 g of dried fine silage sample of smaller than 1mm was transferred into heat sealed F57 filter bags of Ankom and were incubated along with four empty bags. The buffer solutions A and B were prepared according to Ankom DaisyII Incubators instruction (Ankom Technology, Macedon, NY, USA). Equal volume of the rumen fluid was obtained from 3 non-lactating Holstein cows (748.0±10 kg) consuming a total mixed ration about 4 hours after morning feeding which was mixed. A maintenance ration (AFRC, 1992) was fed in equal portions twice a day (07:00 and 19:00) consisting of 500 g/kg silage (1:1 maize silage: sweet sorghum silage), 350 g/kg chopped alfalfa and 150 g/kg concentrate. The rumen fluid was immediately transported to the laboratory in a carbon dioxide flask and mixed using a kitchen blender for 30-60 seconds under anaerobic conditions. The fluid was then filtered through four layers of cheesecloth. Each Ankom jar contained 400 ml filtered rumen fluid, 20 bags (four subsample for each sample), 266 ml B solution (15 g Na2CO3, 1 g Na2S.9H2O per liter), 1330 ml A solution (10 g KH2PO4, 0.5 g MgSO4.7H2O, 0.5 g NaCl, 0.1 g CaCl2.2H2O and 0.5 g urea per liter) at pH=6.8. The jars were then placed in the Ankom DaisyII device for 48 h at 39.5°C. At completion of incubation, the jars were removed and the fluid was drained. The bags were rinsed thoroughly with cold tap water with minimal mechanical agitation until the water was clear. The rinsed bags were transferred into the Ankom200 Fiber Analyzer, aNDF was determined based on the ANKOM protocol. The bags were dried at 60°C for 48 h and IVD was calculated.
Gas production
The in vitro gas accumulation was measured as described by Weimer et al. (2005). Approximately 200 mg of each sample was weighed into the graduated glass syringes of 100 ml. Three vials were placed as control (containing 30 ml mixture of rumen fluid and artificial saliva and no sample) in the beginning, middle and end of vial rows. Solution of micro mineral (13.2 g CaCl2.2H2O, 10 g MnCl2.4H2O, 1 g CoCl2.6H2O, 8 g FeCl3.6H2O per 100 ml solution), rumen buffer (4 g NH4HCO3, 35 g NaHCO3 per 1 litre of solution), macro mineral (5.7 g Na2HPO4, 6.2 g KH2PO4, 0.6 g MgSO4.7H2O per 1 litre of solution), resazurine (1 g per 1 litre) and regenerative (4 ml NaOH 1 N, 625 mg Na2S.9H2O and 95 ml distilled water) were prepared. The rumen fluid was collected and filtered from three ruminally fistulated non-lactating Holstein cows and was used for estimating in vitro true DM digestibility.
All procedures of handling rumen fluid were under continuous flow of CO2. To vials, 10 ml rumen fluid and 20 ml buffer solution were added. Mixture was placed in a shaking water bath at 39.0±0.5°C for 30 min after the start of incubation. Rubber stopper was sealed with a light coating of petrolatum and vials were capped with butyl rubber stoppers, sealed with aluminium crimps. Gas pressures were measured with a digital pressure gauge (UniMano 1000, NeTech, USA) and the gas production (GP) was recorded at 2, 4, 6, 8, 12, 24, 36, 48, 72 and 96 hours of incubation. The amount of GP was corrected for blanks and gas production was fitted with the following model (Ørskov and McDonald, 1979):
Y=b (1-e-ct). Where, b is the GP from the digestible fraction (ml), c is the GP rate constant (/h), t is incubation time (h), Y is gas production at time t.
ME, OMD and NEl of samples using GP at 24 hours were estimated using equations described by Close and Menke (1986), Menke et al. (1979) and Menke and Steingass (1988), respectively:
ME (MJ/kg DM) =1.06 + (0.157×GP24) + (0.0084×CP) + (0.022×EE) + (0.0081×CA)
OMD (g/kg DM) =148.8 + (8.89×GP24) + (0.45×CP) + (0.0651×CA)
NEl (MJ/kg DM) = 0.54+ (0.0959×GP24) + (0.0038×CP) + (0.001733×EE)
Where, GP is gas production (ml/200 mg DM), GP24 is net gas production (ml/200 mg DM) at 24 h of incubation, CP is crude protein (g/kg DM), EE is ether extract (g/kg DM), CA is crude ash (g/kg DM).
Statistical analysis
Data were analyzed using the GLM of Statistical Analysis System (SAS, 2003). Orthogonal contrasts were used to test linear, quadratic and cubic effects of ensiling time. The statistical significance level was considered as P< 0.05.
Results
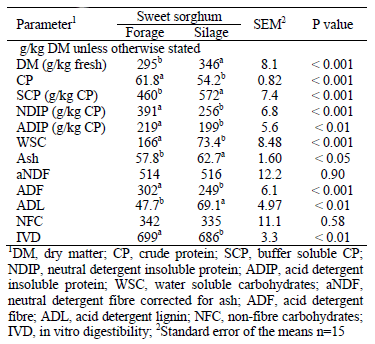
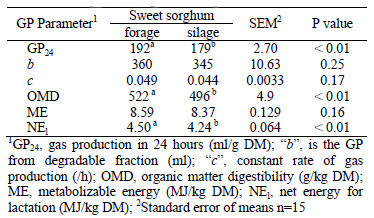
Ensiled material had significantly higher DM, SCP, ash and ADL concentrations than original forage (Table 1). On the other hand, fresh material contains comparatively higher CP, NDIP, ADIP, WSC, ADF, IVD (Table 1) and OMD concentration and NEl content (Table 4) than silage (P< 0.01).
Table 1: Chemical composition of sweet sorghum forage and silage
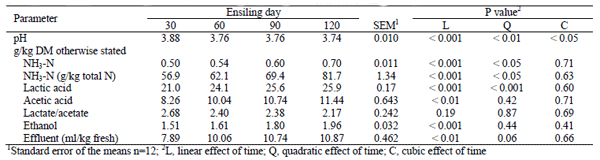
A quadratic decreasing (P< 0.01) trend in silage pH with increasing ensiling time was observed (from 3.88 to 3.74 for 30 and 120 days of ensiling, respectively). The concentrations of NH3-N, lactic acid, acetic acid and ethanol increased (P< 0.01) as the ensiling time advanced from 30 to 120 days (Table 2). But, propionic and butyric acids were not detected in sweet sorghum silages.
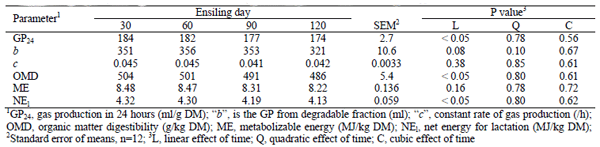
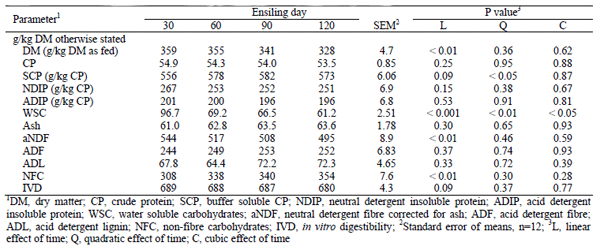
A linear decrease (P< 0.01) of DM, WSC and aNDF with prolonged ensiling time of sweet sorghum was observed (Table 3). A significantly highest (quadratic trend) buffer SCP concentration in 90 days silage was observed (Table 3). The NFC concentration of fresh sweet sorghum decreased non-significantly during the first 30 days of ensiling (342 versus 308 g/kg DM), but increased to 354 g/kg DM by days 120 of ensiling. On contrary, prolonging the ensiling time decreased linearly (P< 0.05) GP24 and NEl content (Table 5).
Discussion
Silage pH is an important parameter in the long term stability of ensiled forages. For instance, forage with low buffering capacity allows a pH drop rapidly even when acid production is small (Alli et al. 1983). A pH below 4.0 is considered satisfactory for long term storage of ensiled material (Jaster, 1995). In this study, propionic or butyric acids were not detected. This may be due to acidic environment unsuitable for the entobacteria and clostridia deleterious activity, but promotes chemical hydrolysis of hemicellulose (McDonald et al. 1991). The decrease in silage pH in this experiment is similar to those reported in previous studies ensiling sweet sorghum by Philipp et al. (2007).
With advancing ensiling time lactic and acetic acids increased. High acetic acid concentration may reflected fermentation of pentose sugars released from hemicellulose fraction to equal proportions of lactic and acetic acids by hetero-fermentative LAB (McDonald et al. 1991). Schmidt et al. (2009) reported that the population of LAB in alfalfa silage (without additives) peaked between 5 and 45 days (> 9 log CFU/g) of ensiling and increased further after 180 days of ensilage. In present study, increasing fermentation end products (lactate, acetate and ethanol) after 3 months of ensiling suggest that microbial activity persists even at low pH condition as claimed by Kung and Der Bedrosian (2010). Lactobacillus buchneri appears to be active for longer duration in corn silage and probably this contributes to the present findings. Under anaerobic conditions and low pH, this organism is able to convert lactic acid to acetic acid, ethanol and 1, 2 propanediol (Oude-Elferink et al., 2001). Silages with high ethanol concentration are an indicative of slow decline in pH of ensiled material and having higher final pH values. Driehuis and Wikselaar (2000) reported that grass silages with 48 to 63 g ethanol/kg DM increase the silage pH more than 5.3.
Table 2: Fermentation characteristics of sweet sorghum silage at different ensiling times
Table 3: Chemical composition of sweet sorghum silage at different ensiling times
Table 4: The gas production parameters of fresh sweet sorghum forage and silage
At ensiling, fresh sweet sorghum forage there was no appreciable quantity of NH3-N. However, the NH3- N concentration increased steadily from 30 to 120 days for sweet sorghum silages. NH3-N is a product of bacterial deamination of amino acids rather than a product of acid hydrolysis of silage VFA (Ohshima & McDonald 1978). Filya (2003) reported increasing amount of NH3-N in corn and sorghum materials through 90 days of ensiling. Kleinschmit and Kung (2006) reported a steady increase in NH3-N in corn silage through 361 d of ensiling without reaching a plateau. Silage is considered as an excellent and good when the NH3-N/TN is below 7 g/100 gTN while and 7- 10 g/100 gTN, respectively (Romero, 2004). As per the criteria, silage ensiled for 90 and 120 days in the present study may be categorized as an excellent and good quality, respectively.
NDF concentration of silage decreased between 30 to 120 days of ensiling which might be attributed to degradation of cell wall by activity of bacterial enzymes (cellulase and hemicellulase) and production of organic acids during fermentation (Yahaya et al., 2001). The NDF of sweet sorghum silages increased after first 30 days of ensiling, but decreased until day 120. Similarly, Henk and Linden (1992) reported that NDF content of sweet sorghum silage increased between day 4 and 7 of fermentation before continuing to decline. The reason of this change in NDF concentration is yet to be documented however, the high WSC content in sweet sorghum may have resulted to rapid decrease in the concentration of WSC in the first 30 day of ensiling (from 166 to 96.7 g/kg DM) which might increase the ratio of NDF to DM.
Table 5: The gas production parameters of sweet sorghum silage at different ensiling times
WSC concentration in silages decreased dramatically by 69.3 g/kg during the first 30 day of fermentation and continued to gradually fall in second 30 day (27.5 g/kg) but after day 60 the decrease was negligible. These data shows the rapid microbial utilization of sugars in early stages of fermentation at low pH. This is confirmed by the continued small reduction in silage pH with increase in ethanol production as mentioned by Stokes and Chen (1994). In addition, the DM content reduced as ensiling progressed. For example, 328 and 359 g/kg of DM were observed in 120 and 30 day sweet sorghum silage preparation. The decrease could be explained by a moderate fall in reducing sugars which is similar to Henk and Linden (1992) and Stokes and Chen (1994) findings for sweet sorghum and corn silage, respectively. This reduction was ascribed to the continued maintenance requirement of the microbial population in the sweet sorghum silage.
The cellulose content (data are not presented) was not affected (P> 0.05; SEM 5.682) by ensiling time while hemicellulose decreased (P< 0.05; SEM 13.63) about 57 g/kg of DM likewise. Morrison (1979) showed that the core lignin concentration of forage did not change and cellulose could decrease to 50 g/kg during ensiling after 150 day of storage. Yahaya et al. (2001) confirmed that considerable loss of the hemicellulose and pectin fractions occurred in alfalfa and orchard grass silage between fresh forage and ensiled materials.
Studies report that the respiratory process accounts greater loss of silage DM and reducing the quality of material(Bolsen, 1995) due to heating and conversion of sugars and organic acids to undesirable products such as NH3, CO2, and H2O (Stokes and Chen, 1994). By contrast, our study observed increase in DM content in the first 30 day of ensiling (from 295 g/kg of fresh forage to 359 g/kg in day 30) and latter decreased linearly to 328 g/kg at day 120.
In our study, ensiling time had no significant effect on IVD of sweet sorghum silages. Similarly, Der Bedrosian et al. (2010) reported that time of ensiling did not affect the in vitro NDF digestibility of two corn silage hybrids between 45 and 315 day of ensiling. Furthermore, the length of storage (up to 180 day) had no effect on the digestibility of cell walls as evaluated by in vitro gas production (Cone et al., 2008). Increased in vitro digestion of starch in normal and brown mid-rib corn silage hybrids through 270 day of storage has been reported by Der Bedrosian et al. (2010). In contrast, increasing the time of ensiling had no affect on starch digestion during in vitro gas production system (Cone et al., 2008). Digestibility is highly influenced by fibre and sugar concentrations in the forage. For instance, the lower NDF: sugars ratio there would be the higher IVD (Rodrigues et al., 2001). Therefore, simultaneous decreases in NDF and sugar contents during the ensiling process resulted to insignificant effect on IVD in current experiment. This is not in agreed with the findings of Pedroso et al. (2005) and Siqueira (2005). They report that ensiling the high WSC content forages such as sugarcane causes a greater loss of WSC, but increases the fibre components and thus reduces IVD in silage.
Conclusion
This study showed that the duration of ensiling changed characteristics of sweet sorghum silage. Despite strong acidic condition, anaerobic activity process continued till the end of our experiment (120 days of ensiling). Therefore, there is no need for silage additives to maintain the silage quality for four months.
Acknowledgement
The authors are grateful to the management board of the Shamim Roshd Espadan Co. and Mr.Changizi for providing materials and monetary fund to undertake this study.
References
AFRC. 1992. Nutritive requirements of ruminant animals: Protein. Nutrition Abstracts and Reviews, (Series B) 62: 787-835.
Alli, I., Fairbairn, R. and Baker, B.E. 1983. The effects of ammonia on the fermentation of chopped sugarcane. Animal Feed Science and Technology, 9: 291-299.
AOAC. 1999. Official Methods of Analysis, Association of Official Analytical Chemists International, Gaithersburg, MD.
Benton, J.R., Klopfenstein, T. and Erickson, G.E. 2005. Effects of corn moisture and length of ensiling on dry matter digestibility and rumen degradable protein. Nebraska Beef Cattle Reports, 31-33. University of Nebraska, Lincoln.
Bolsen, K.K. 1995. Silage: Basic principles. PP: 163-176. In: Barnes, R.F., Miller, D.A. and Nelson, C.J. (Eds.), Forage Vol. II, The science of grassland agriculture, 5th ed. Iowa State University Press, Ames, IA.
Calabrò, S., Tudisco, R., Grossi, M., Bovera, F., Cutrignelli, M.I., Guglielmelli, A., Piccolo, V. and Infascelli, F. 2007. In vitro fermentation characteristics of corn and sorghum silages. Italian Journal of Animal Science, 6: 559-562.
Caswell, L.F., Kalmbacher, R.S. and Martin, F.G. 1983. Yield and silage fermentation characteristics of corn, sweet sorghum, and grain sorghum. Proceedings-Soil and Crop Science Society of Florida. 42: 139-142.
Close, W.H. and Menke, K.H. 1986. Evaluation on the basis of metabolizable energy. In: Deutsche Stiftung für Internationale Entwicklung, Zentralstelle für Ernährung und Landwirtschaft (Ed.), Selected Topics in Animal Nutrition. A Manual Prepared for the 3rd Hohenheim Course on Animal Nutrition in the Tropics and Semi-Tropics, Second ed. Feldafing, Germany, pp: 60-63.
Colombo, D., Crovetto, G.M., Colombini, S., Galassi, G. and Rapetti, L. 2007. Nutritive value of different hybrids of sorghum forage determined in vitro. Italian Journal of Animal Science, 6: 289-291.
Cone, J.W., Van Gelder, A.H., Van Schoten, H.A. and Groten, J.A.M. 2008. Effects of chop length and ensiling period of forage maize on in vitro rumen fermentation characteristics. Netherlands Journal of Agricultural Science, 55: 155-166.
Der Bedrosian, M.C., Kung Jr, L. and Nestor Jr, K.E. 2010. The effects of length of storage on the composition and nutritive value of corn silage. Journal of Dairy Science, 93: 176. Abstr.
Driehuis, F. and Van Wikselaar, P.G. 2000. The occurrence and prevention of ethanol fermentation in high-dry-matter grass silage. Journal of the Science of Food and Agriculture, 80: 711-718.
Filya, I. 2003. The effect of Lactobacillus buchneri and Lactobacillus plantarum on the fermentation, aerobic stability, and ruminal degradability of low dry matter corn and sorghum silage. Journal of Dairy Science, 86: 3575-3581.
Gary, M. 1992. Ensiling process. In: Silage Manual. Bjorge M. and Bjorge, H. (Eds.). Edmonton, Alberta, pp: 14-17.
Henk, L.L. and Linden, J.C. 1992. Simultaneous ensiling and enzymatic hydrolysis of structural polysaccharides. Enzyme and Microbial Technology, 14: 923-930.
Hoffman, P.C., Esser, N.M., Shaver, R.D., Coblentz, W.K., Scott, M.P., Bodnar, A.L., Schmidt, R.J. and Charley, R.C. 2011. Influence of ensiling time and inoculation on alteration of the starch-protein matrix in high-moisture corn. Journal of Dairy Science, 94: 2465-2474.
Ishler, V. and Varga, G. 2001. Carbohydrate nutrition for lactating dairy cattle. Pennsylvania State University, Code #: DAS 01-29, pp: 1-11.
Jaster, E.H. 1995. Legume and grass silage preservation. pp. 91-115. In: Moore, K.J., Peterson, M.A. (Eds.), Post-harvest physiology and preservation of forages. CSSA-ASA, Madison, WI.
Kleinschmit, D.H. and Kung Jr, L. 2006. The effects of Lactobacillus buchneri 40788 and Pediococcus pentosaceus R1094 on the fermentation of corn silage. Journal of Animal Science, 89: 3999-4004.
Kung Jr, L. and Der Bedrosian, M. 2010. How well do we really understand silage fermentation? Proceedings 2010 Cornell Nutrition Conference for Feed Manufacturers. October 19-21. Doubletree Hotel East Syracuse, New York. Department of Animal Science at the New York State College of Agriculture and Life Sciences (A Statutory College of the State University of New York) Cornell University Ithaca, New York, pp: 87-93.
Linden, J.C., Henk, L.L., Murphy, V.G., Smith, D.H., Gabrielsen, B.C., Tengerdy, R.P. and Czako, L. 1987. Preservation of potential fermentables in sweet sorghum by ensiling. Biotechnology and Bioengineering, 30: 860-867.
Licitra, G., Hernandez, T.M. and Van Soest, P.J. 1996. Standardization of procedures for nitrogen fractionation of ruminant feeds. Animal Feed Science and Technology, 57: 347-358.
Masuko, T., Minami, A., Iwasaki, N., Majima, T., Nishimura, SI. and Lee, Y.C. 2005. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Analytical Biochemistry, 339: 69- 72.
McDonald, P., Henderson, A.R. and Heron, S.J.E. 1991. The biochemistry of silage, 2nd ed. Chalcombe Publications, Marlow, Buckinghamshire, UK.
Megias, M.D., Martinez-Teruel, A., Gallego, J.A. and Nunez, J.M. 1993. Chemical changes during ensiling of orange peel. Animal Feed Science and Technology, 43: 269-274.
Menke, K.H., Raab, L., Salewski, A., Steingass, H., Fritz, D. and Schneider, W. 1979. The estimation of the digestibility and metabolisable energy content of ruminant feedstuffs from the gas production when they are incubated with rumen liquor. Journal of Agricultural Science, 93: 217-222.
Menke, K.H. and Steingass, H. 1988. Estimation of the energetic feed value obtained from chemical analyses and gas production using rumen fluid. Animal Research and Development, 28: 7-55.
Morrison, I.M. 1979. Changes in the cell wall components of laboratory silages and the effect of various additives on these changes. Journal Agricultural Science Cambridge, 93: 581-586.
Newbold, J.R., Lewis, E.A., Lavrijssen, J., Brand, H.J., Vedder, H. and Bakker, J. 2006. Effect of storage time on ruminal starch degradability in corn silage. Journal of Dairy Science 89: 190. Abstr.
Ohshima, M. and McDonald, P. 1978. A review of the changes in nitrogenous compounds of herbage during ensilage. Journal of the Science of Food and Agriculture, 29: 497-505.
Oude Elferink, S.J.W.H., Krooneman, J., Gottschal, J.C., Spoelstra, S.F., Faber, F. and Driehuis, F. 2001. Anaerobic conversion of lactic acid to acetic acid and 1, 2-propanediol by Lactobacillus buchneri. Applied and Environmental Microbiology, 67: 125-132.
Ørskov, E.R. and McDonald, I. 1979. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. Journal Agricultural Science Cambridge, 92: 499-503.
Pedroso, A.F., Nussio, L.G., Paziani, S.F., Loures, D.R.S., Igarasi, M.S., Coelho, R.M., Packer, I.H., Horii, J. and Gomes, L.H. 2005. Fermentation and epiphytic microflora dynamics in sugar cane silage. Scientia Agricola, 62, : 427-432.
Philipp, D., Moore, K.J., Pedersen, J.F., Grant, R.J., Redfearn, D.D. and Mitchell, R.B. 2007. Ensilage performance of sorghum hybrids varying in extractable sugars. Biomass and Bioenergy, 31: 492- 496.
Playne, M.J. 1985. Determination of ethanol, volatile fatty acids, lactic and succinic acids III fermentation liquids by gas chromatography. Journal of the Science of Food and Agriculture, 36: 638-644.
Rodrigues, A.A., Cruz, G.M.C., Batista, L.A.R. and Landell, M.G.A. 2001. Qualidade de dezoito variedades de cana-de-açúcar como alimento para bovinos. In: Reuniao da sociedade brasileira de zootecnia, 38., Piracicaba, 2001. Anais. Piracicaba: SBZ, pp:1111-1112.
Romero, L.A. 2004. Ensilaje de soja, calidad en forrajes conservados. Manual de actualización técnica. Merco Láctea, San Francisco, Córdova, Argentina, pp: 40- 41.
SAS, 2003.User’s Guide.Statistical Analysis System Institute Inc., Cary, NC, USA.
Schmidt, R.J., Hu, W., Mills, J.E.A. and Kung Jr, L. 2009. The development of lactic acid bacteria and Lactobacillus buchneri and their effects on the fermentation of alfalfa silage. Journal of Dairy Science, 92: 5005-5010.
Sipos, B., Réczey, J., Somorai, Z., Kádár, Z., Dienes, D and Réczey, K. 2009. Sweet Sorghum as Feedstock for Ethanol Production: Enzymatic Hydrolysis of Steam-Pretreated Bagasse. Applied Biochemistry and Biotechnology, 153: 151-162.
Siqueira, G.R. 2005. Cana-de-açúcar (Saccharum officinarum L.) ensilada com aditivos químicos e bacterianos. Jaboticabal: UNESP/FCAV Dissertação (Mestrado). pp: 91.
Stokes, M.R. and Chen, J. 1994. Effects of an enzymeinoculant mixture on the course of fermentation of corn silage. Journal of Dairy Science, 77: 3401-3409.
Van Soest, P.J. and Wine, R.H. 1968. Determination of lignin and cellulose in acid-detergent fiber with permanganate. Journal of the Association of Official Analytical Chemists, 51: 780-785.
Van Soest, P.J. 1973. Collaborative study of aciddetergent fiber and lignin. Journal of the Association of Official Analytical Chemists, 56: 781-784.
Van Soest, P.J., Robertson, J.B. and Lewis, B.A. 1991. Methods for dietary fiber, neutral fiber and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science, 74: 473-481.
Ward, R.T. and de Ondarza, M.B. 2008. Effect of month of sample submitted on corn silage nutrient fractions, starch availability, NDF digestibility, and fermentation profiles measured at a commercial forage-testing laboratory. Journal of Dairy Science, 91: 30 (Abstr).
Weimer, P.J., Dien, B.S., Springer, T.L. and Vogel, K.P. 2005. In vitro gas production as a surrogate measure of the fermentability of cellulosic biomass to ethanol. Applied Microbiology and Biotechnology, 67: 52-58.
Yahaya, M.S., Kimura, A., Harai, J., Nguyen, H.V., Kawai, M., Takahashi, J. and Matsuoka, S. 2001. Effect of length of ensiling on silo degradation and digestibility of structural carbohydrates of lucerne and orchardgrass. Animal Feed Science and Technology, 92: 141-148.
Yahaya, M.S., Kawai, M., Takahashi, J. and Matsuoka, S. 2002. The effects of different moisture content and ensiling time on silo degradation of structural carbohydrate of orchardgrass. Asian-Australasian Journal of Animal Sciences, 15: 213-217.







.jpg&w=3840&q=75)