From colostrum to weaning: nutritional regulation of gut function in the dairy calf
Raising healthy and productive calves is fundamental in ensuring the long-term success of the Canadian dairy industry. Unfortunately, pre-weaning calves suffer from the highest rates of morbidity (34%) and mortality (5%) amongst all animals on the dairy farm. Digestive disorders occurring before 2 weeks of age are the largest contributor to calf sickness and death; however, a multitude of previous research has demonstrated that proper nutritional management can have a positive impact on gut function and development, as well as productivity and health. During the first day of life, ensuring passive transfer in newborn calves is essential. Yet, colostrum contains an abundance of bioactive compounds aside from IgG, such as oligosaccharides, fatty acids and growth hormones, that may have beneficial effects on early life gut development. Maximizing preweaning whole milk or milk replacer (MR) intake is essential in promoting animal health and elfare, but there is growing interest in feeding elevated levels of MR as the macronutrient composition, namely lactose and fat, differs greatly from whole milk. Moreover, weaning calves from enhanced milk feeding programs can often result in health and production challenges and the source and level of starch in calf starter may further exacerbate this issue. Further research regarding optimal nutritional strategies during the newborn, pre-weaning and weaning stages is needed to allow industry representatives and dairy producers to make confident decisions to promote calf health, welfare and productivity.
Bach, A., L. Domingo, C. Montoro, and M. Terré. 2013. Short communication: insulin responsiveness is affected by the level of milk replacer offered to young calves. J. Dairy Sci. 96:4634–4637.
Ballou, M.A., D.L. Hanson, C.J. Cobb, B.S. Obeidat, M.D. Sellers, A.R. Pepper-Yowell, J.A. Carroll, T.J. Earleywine, and S.D. Lawhon. 2015. Plane of nutrition influences the performance, innate leukocyte responses and resistance to an oral Salmonella enterica serotype Typhimurium challenge in Jersey calves. J. Dairy Sci. 98(3):1972-1982.
Beam, A.L., J.E. Lombard, C.A. Kopral, L.P. Garber, A.L. Winter, J.A. Hicks, and J.L. Schlater. 2009. Prevalence of failure of passive transfer of immunity in newborn heifer calves and associated management practices on US dairy operations. J. Dairy Sci. 92:3973-3980.
Berends, H., J. J. van den Borne, N. Stockhofe-Zurwieden, M. S. Gilbert, T. Zandstra, W. F. Pellikaan, C. G. van Reenen, E. A. M. Bokkers, and W. J. J. Gerrits. 2015. Effects of solid feed level and roughage-to-concentrate ratio on ruminal drinking and passage kinetics of milk replacer, concentrates, and roughage in veal calves. J. Dairy Sci. 98:5621–5629.
Berge, A.C.B., T.E. Besser, D.A. Moore, and W.M. Sisco. 2009. Evaluation of the effects of oral colostrum supplementation during the first fourteen days on the health and performance of preweaned calves. J. Dairy Sci. 92:286-295.
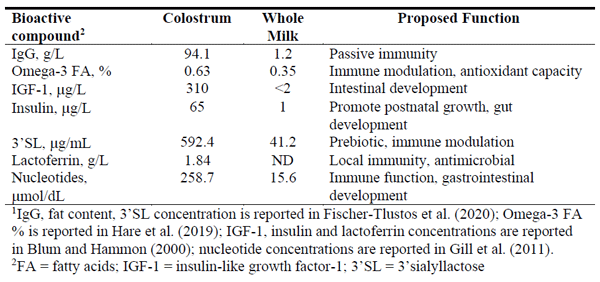
Blum, J.W. and H. Hammon. 2000. Colostrum effects on the gastrointestinal tract, and on nutritional, endocrine and metabolic parameters in neonatal calves. Live. Prod. Sci. 66:151-159.
Burrin, D.G., B. Stoll, and X. Guan. 2003. Glucagon-like peptide-2 function in domestic animals. Domest. Anim. Endocrinol. 24:103-122.
Connor, E. E., R. L. Baldwin, C. Li, R. W. Li, and H. Chung. 2013. Gene expression in bovine rumen epithelium during weaning identifies molecular regulators of rumen development and growth. Funct. Integr. Genomics.13:133–142.
Davis, C.L. and J.K. Drackley. 1998. The development, nutrition, and management of the young calf. Iowa State Univ. Press, Ames.
DeNise, S.K., J.D. Robison, G.H. Stott, and D.V. Armstrong. 1989. Effects of passive immunity on subsequent production in dairy heifers. J. Dairy Sci. 72:552–554.
Dennis, T.S., F.X. Suarez-Mena, T.M. Hill, J.D. Quigley, R.L. Schlotterbeck, R.N. Klopp, G.J. Lascano, and L. Hulbert. 2018. Effects of gradual and later weaning ages when feeding high milk replacer rates on growth, textured starter digestibility, and behavior in Holstein calves from 0 to 4 months of age. Journal of dairy science. 101(11):9863-75.
de Paula Vieira, A., Guesdon, V., de Passillé, A.M., von Keyserlingk, M.A.G., and Weary, D.M. 2008. Behavioural indicators of hunger in dairy calves. Appl. Anim. Behav. Sci. 109: 180–189.
Desjardins-Morrissette, M., J.K. van Niekerk, D. Haines, T. Sugino, M. Oba, and M.A. Steele. 2018. The effect of tube vs. bottle feeding colostrum on IgG absorption, abomasal emptying and plasma hormone concentrations in newborn calves. J. Dairy Sci. 101:4168–4179.
Eckert, E., H. E. Brown, K. E. Leslie, T. J. DeVries, and M. A. Steele. 2015. Weaning age effects growth, feed intake, gastrointestinal development, and behaviour in Holstein calves fed an elevated plane of nutrition during the preening stage. J. Dairy Sci. 98:6315-6326.
Ellingsen, K., C.M. Mejdell, N. Ottesen, S. Larsen, and A.M. Grondahl. 2016. The effect of large milk meals on digestive physiology and behaviour in dairy calves. Physiol. Behav. 154:169–174.
Faber, S.N., N.E. Faber, T.C. McCauley, and R.L. Ax. 2005. Effects of colostrum ingestion on lactational performance. Prof. Anim. Sci. 21:420-425.
Fischer, A.J., Y. Song, Z. He, D.M. Haines, L.L Guan, and M.A. Steele. 2018a. Effect of delaying colostrum feeding on passive transfer and intestinal bacterial colonization in neonatal male Holstein calves. J. Dairy Sci. 101:3099-3109. https://doi.org/ 10.3168/jds.2017-13397
Fischer, A. J., N. Malmuthuge, L. L. Guan, and M. A. Steele. 2018b. Short communication: The effect of heat treatment of bovine colostrum on the concentration of oligosaccharides in colostrum and in the intestine of neonatal male Holstein calves. J. Dairy Sci. 101:401-407.
Fischer-Tlustos, A.J., K. Hertogs, J.K. Van Niekerk, M. Nagorske, D.M. Haines, and M.A. Steele. 2020. Oligosaccharide concentrations in colostrum, transition milk, and mature milk of primi- and multi-parous Holstein cows during the first week of lactation. J. Dairy Sci. In Press: Accepted Dec 6, 2019.
Fukumori, R. T., T. Mita, T. Sugino, T. Obitsu, and K. Taniguchi. 2012. Plasma concentrations and effects of glucagon-like peptide-1 (7-36) amide in calves before and after weaning. Domest. Anim. Endocrinol. 43:299-306.
Gelsinger, S.L., Heinrichs, A.J., and Jones, C.M. 2016. A metaanalysis of the effects of preweaned calf nutrition and growth on first-lactation performance. J. Dairy Sci. 99(8):6206–6214.
Gill, B.D., H.E. Indyk, and M. Manley-Harris. 2011. Determination of total potentially available nucleosides in bovine milk. Int. Dairy J. 21:34–41.
Godden, S.M., J.P. Fetrow, J.M. Freitag, L.R. Green, and S.J. Wells. 2005. Economic analysis of feeding pasteruized non-salable milk versus conventional milk replacer to dairy calves. J. Am. Vet. Med. Assoc. 226:1547-1554.
Haisan, J., M.A. Steele, D.J. Ambrose, and M. Oba. 2019. Effects of amount of milk fed, and starter intake, on performance of group-housed dairy heifers during the weaning transition. Appl. Anim. Sci. 35:88–93.
Hare, K.S., K. Hertogs, A. Fischer, P. Vahmani, M.E.R. Dugan, and M. Steele. 2019. Omega-3 and -6 fatty acids are more abundant in colostrum than transition and whole milk. J. Dairy Sci. 102(Suppl. 1):154.
Hill, T.M., J.D. Quigley, H.G. Bateman II, F.X. Suarez-Mena, T.S. Dennis, and R.L. Schlotterbeck. 2016. Effect of milk replacer program on calf performance and digestion of nutrients in dairy calves to 4 months of age. J. Dairy Sci. 99(10):8103-10.
Inabu, Y., A. Fischer, Y. Song, L.L. Guan, M. Oba, M.A. Steele, and T. Sugino. 2018. Short communication: The effect of delayed colostrum feeding on plasma concentrations of glucagonlike peptide 1 and 2 in newborn calves. J. Dairy Sci. 101:6627-6631.
Inabu, Y., J. Pyo, S. Pletts, L.L. Guan, M.A. Steele, and T. Sugino. 2019. Effect of extended colostrum feeding on plasma glucagon-like peptide-1 concentration in newborn calves. J. Dairy Sci. 102:4619–4627.
Jenkins, K.J., J.K.G. Kramer, F.D. Sauer, and D.B. Emmons. 1985. Influence of triglycerides and free fatty acids in milk replacers on calf performance, blood plasma, and adipose lipids. J. Dairy Sci. 68:669–680.
Khan, M. A., H. J. Lee, W. S. Lee, H. S. Kim, S. B. Kim, K. S. Ki, J. K. Ha, H. G. Lee, and Y. J. Choi. 2007a. Pre- and postweaning performance of Holstein female calves fed milk through stepdown and conventional methods. J. Dairy Sci. 90:876–885.
Khan, M. A., H. J. Lee, W. S. Lee, H. S. Kim, K. S. Ki, T. Y. Hur, G. H. Suh, S. J. Kang, and Y. J. Choi. 2007b. Structural growth, rumen development, and metabolic and immune responses of Holstein male calves fed milk through step-down and conventional methods. J. Dairy Sci. 90:3376–3387.
Li, R.W., E.E. Connor, C. Li, R.L. Baldwin VI, and M.E. Sparks. 2012. Characterization of the rumen microbiota of pre-ruminant calves using metagenomics tools. Environ. Microbiol. 14:129-139.
Ma, T., E. O’Hara, Y. Song, A. Fischer, Z. He, M.A. Steele, and L.L. Guan. 2019. Altered mucosa-associated microbiota in the ileum and colon of neonatal calves in response to delayed first colostrum feeding. J. Dairy Sci. 102:7073-7086.
MacPherson, J., S.J. Meale, K. Macmillan, J. Haisan, C.J. Bench, M. Oba, and M.A. Steele. 2018. Effects of feeding frequency of an elevated plane of milk replacer and calf age on behavior, and glucose and insulin kinetics in male Holstein calves. Animal. 13:1385–1393.
Malmuthuge, N., M. Li, L. A. Goonewardene, M. Oba, and L. L. Guan. 2013. Effect of calf starter feeding on gut microbial diversity and expression of genes involved in host immune responses and tight junctions in dairy calves during weaning transition. J. Dairy Sci. 96:189-200.
Malmuthuge, N., Y. Chen, G. Liang, L.A. Goonewardene, and L.L. Guan. 2015. Heat-treated colostrum feeding promotes beneficial bacteria colonization in the small intestine of neonatal calves. J. Dairy Sci. 98:8044-8053.
McGuirk, S.M. and M. Collins. 2004. Managing the production, storage and delivery of colostrum. Vet. Clin. North Am. Food Anim. Pract. 20:593-603.
Meale, S. J., L. N. Leal, J. Martín-Tereso, and M. A. Steele. 2015. Delayed weaning of Holstein bull calves fed an elevated plane of nutrition impacts feed intake, growth and potential markers of gastrointestinal development. Anim. Feed Sci. Tech. 209:268–273.
Meale, S. J., S. C. Li, P. Azevedo, H. Derakhshani, T. J. DeVries, J. C. Plaizier, M. A. Steele, and E. Khafipour. 2017. Weaning age influences the severity of gastrointestinal microbiome shifts in dairy calves. Sci. Rep. 7: doi:10.1038/s41598-017-00223-7.
Opgenorth, J., L.M. Sordillo, and M.J. VandeHaar. 2019. Colostrum supplementation with omega-3 fatty acids and ɑ-tocopherol decreases indicators of oxidative stress and alters plasma fatty acid profile in newborn calves during the first week of life. J. Dairy Sci. 102(Suppl. 1):86.
Pakkanen, R. and J. Aalto. 1997. Growth factors and antimicrobial factors of bovine colostrum. Int. Dairy J. 7(5):285-297.
Pyo, J., K. Hare, S. Pletts, Y. Inabu, D. Haines, T. Sugino, L. L. Guan, and M. Steele. 2020. Feeding colostrum or a 1:1 colostrum:milk mixture for 3 d postnatal increase small intestinal development and minimally influences plasma GLP-2 and serum IGF-1 concentrations in Holstein bull calves. J. Dairy Sci. In Press: Accepted 01/04/2020.
Reinhardt, V. and A. Reinhardt. 1981. Natural sucking performance and age at weaning in zebu cattle (Bos indicus). J. Agric. Sci. 96:309–312.
Robison, J.D., G.H. Stott, and S.K. DeNise. 1988. Effects of passive immunity on growth and survival in the dairy heifer. J. Dairy Sci. 71:1283-1287.
Round, J.L. and S.K. Mazmanian. 2009. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9:313-323.
Russel, S.L., M.J. Gold, B.P. Willing, L. Thorson, K.M. McNagny, and B.B. Finlay. 2013. Perinatal antibiotic treatment affects murine microbiota, immune responses and allergic asthma. Gut Microbes. 4:158-164.
Shivley, C.B., J.E. Lombard, N.J. Urie, D.M. Haines, R. Sargent, C.A. Kopral, T.J. Earleywine, J.D. Olson, and F.B. Garry. 2018. Preweaned heifer management on US dairy operations: Part II. Factors associated with colostrum quality and passive transfer status of dairy heifer calves. J. Dairy Sci. 101:9185-9198.
Soberon, F., E. Raffrenatio, R.W. Everett, and M.E. van Amburgh. 2012. Preweaning milk replacer intake and effects on long-term productivity of dairy calves. J. Dairy Sci. 95:783-793.
Tamate, H., R. Getty, A. D. McGilliard, and N. L. Jacobson. 1962. Effect of various dietaries on anatomical development of stomach in calf. J. Dairy Sci. 45:408-420.
Taylor-Edwards, C.C., D.G. Burrin, J.J. Holst, K.R. McLeod, and D.L. Harmon. 2011. Glucagon-like peptide-2 (GLP-2) increases small intestinal blood flow and mucosal growth in ruminating calves. J. Dairy Sci. 94:888-898.
Terré, M., M. Devant, and A. Bach. 2007. Effect of level of milk replacer fed to Holstein calves on performance during the preweaning period and starter digestibility at weaning. Livest. Sci. 110:82–88.
Urie, N.J., J.E. Lombard, C.B. Shivley, C.A. Kopral, A.E. Adams, T.J. Earleywine, J.D. Olson, and F.B. Garry. 2018a. Preweaned heifer management on US dairy operations: Part V. Factors associated with morbidity and mortality in preweaned dairy heifer calves. J. Dairy Sci. 101:9229–9244.
Urie, N.J., J.E. Lombard, C.B. Shivley, C.A. Kopral, A.E. Adams, T.J. Earleywine, J.D. Olson, and F.B. Garry. 2018b. Preweaned heifer management on US dairy operations: Part I. Descriptive characteristics of preweaned heifer raising practices. J. Dairy Sci. 101:9168-9184.
Van Niekerk, J.K., A.J. Fischer-Tlustos, L.L. Diekun, J.D. Quigley, T. Dennis, X. Suarez-Mena, T.M. Hill, R. Schlotterbeck, L.L. Guan, and M.A. Steele. 2020. Impact of amount of milk replacer fed and the processing of corn in starter on growth performance, nutrient digestibility and rumen and fecal fibrolytic bacteria of dairy calves. J. Dairy Sci. 103(3):2186-2199.
Vasseur, E., F. Borderas, R.I. Cue, D. Lefebvre, D. Pellerin, J. Rushen, K.M. Wade, and A.M. de Passille. 2010. A survey of dairy calf management practices in Canada that affect animal welfare. J. Dairy Sci. 93:1307–1315.
Warner, R. G., W. P. Flatt, and J. K. Loosli. 1956. Dietary factors influencing the development of the ruminant stomach. Agric. Food Chem. 4:788–801.
Weaver, D.M., J.W. Tyler, D.C. VanMetrem, D.E. Hostetler, and G.M. Barrington. 2000. Passive transfer of colostral immunoglobulins in calves. J. Vet. Intern. Med. 14:569-577.
Welboren, A., B. Hatew-Chuko, L. Leal, J. Martín-Tereso, and M. Steele. 2019a. Effects of a high lactose milk replacer on glucose metabolism in neonatal calves. WCDS Proc. 31:236.
Welboren, A.C., B. Hatew, L. Leal, J. Martin-Tereso, and M.A. Steele. 2019b. 80 Effects of macronutrient composition of milk replacer on body composition and intestinal development in neonatal dairy calves. J. Anim. Sci. 97(Suppl. 3):70–71.
Welboren, A.C., L.N. Leal, M.A. Steele, M.A. Khan, and J. Martín-Tereso. 2019c. Performance of ad libitum fed dairy calves weaned using fixed and individual methods. Animal. 1-8.
Wilms, J., H. Berends, and J. Martin-Tereso. 2019. Hypertonic milk replacers increase gastrointestinal permeability in healthy dairy calves. J. Dairy Sci. 102:1237–1246.
Winder, C.B., C.A. Bauman, T.F. Duffield, H.W. Barkema, G.P. Keefe, J. Dubuc, F. Uehlinger, D.F. Kelton. 2018. Canadian National Dairy Study: Heifer calf management. J. Dairy Sci. 101:10565-10579.
Yu, Z-T., C. Chen, and D.S. Newburg. 2013. Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology. 23(11):1281-1292.
Nice article - colostrum feeding is extremely important to pass the immunity from mother cows to newborn calves.







.jpg&w=3840&q=75)