Distillers Grains & Milk Fat Depression
Published: June 27, 2012
By: Alvaro Garcia, Dairy Specialist, DVM, Ph.D., Fernando Diaz Royon, DVM (South Dakota State University)
Fat is the milk component most easily modified by the diet with possible changes of up to 3 percentage units (Jenkins, 2007). Nutrition represents the environmental factor with the greatest impact on milk fat and it is a tool that can be used to alter its fatty acid composition. Milk fat depression (MFD) occurs when specific diets cause reduction in milk fat concentration and yield, in addition of changing is fatty acid (FA) composition (Bauman and Griinari, 2001). Least-cost formulation of dairy cattle rations usually calls for maximum inclusion of distillers grains (DG). From a nutritional standpoint however, to achieve adequate nutrient balance the maximum suggested inclusion level for distillers grains has been 20% of the diet dry matter (DM; Schingoethe. 2009). In addition, there is agreement between dairy producers and their nutritionists that the high fat concentration in DG can result in MFD (Diaz-Royon et al., 2011). Due to the high fiber content in DG, dairy nutritionists could be tempted to replace part of the forage neutral detergent fiber (NDF) with NDF from DG. Due to its small particle size this fiber in DG is not "effective", having a reduced capacity to stimulate cud chewing and rumination which would help maintain milk fat (Schingoethe et al., 2009). These factors can lead to rumen acidosis, which can compound the negative effects caused by the rumen load of polyunsaturated fatty acids (PUFA). These effects have been reported in trials conducted by the Dairy Science Department at South Dakota State University. Ciriac et al. (2005) reported a linear decrease in milk fat concentration (3.34 to 2.85%) when DDGS (dried distillers grains with solubles) substituted 0, 7, 14 and 21% of the corn silage, even though the NDF content in the diets remained constant at 32%; milk production in this trial however was unaffected.
Effective fiber
Rumination is the process by which rumen contents are thoroughly mixed and by which the larger, less digested particles are regurgitated, chewed, and swallowed again. This cycle is repeated systematically when new, longer feed particles are ingested by the cow. During rumination, the protective cuticle cover of plant fragments is macerated and the rumen microorganisms can access the highly digestible plant cell contents. Feed particles escape this cycle once they become smaller and denser enough through sinking to the bottom of the rumen and/ or progressing down the digestive tract. In addition to mechanically breaking the forage into smaller particles, chewing stimulates the production of saliva. This saliva is essential due to its high bicarbonate content which neutralizes the continuous rumen acid conditions produced by the microbial fermentation. Structural carbohydrates (e.g. NDF) are instrumental in the physical stimulus that initiates rumination. It is this feed fraction that forms the floating rumen mat which plays an important role in maintaining rumen health and function. This mat scratches the rumen walls contributing the physical stimulus needed for the rumen to contract and originate each rumination cycle. When the particles ingested are too small the formation of this rumen mat is impaired. In the absence of an adequate mat, rumen motility is impaired, rumen retention time decreases, and so does total tract digestibility of the diet. The rumen mat could also be compared with a "forage sieve" that slows particles down long enough for them to be degraded in the rumen by the microorganisms. When rumen contractions are reduced so is cud chewing and consequently less saliva is produced with a reduction in the buffering capacity of the rumen contents. Dietary NDF concentration is poorly related to this "fiber effectiveness". What is really important is not the NDF concentration in the diet alone, but also the particle size of the forage from which this NDF came from. Increased dietary forage NDF increases rumen pH as a result of increased chewing activity and saliva production. It has long been demonstrated that as rumen pH decreases so does milk fat. Research has shown that with rumen pH above 6.0, milk fat percent in Holsteins was 3.5 or greater (Grant et al. 1990). Also, low rumen pH is one major factor that can lead to a change in rumen biohydrogenation (BH) pathways resulting in increased formation of trans-10, cis-12 CLA (conjugated linoleic acid) and related intermediates (Lock y col., 2006).
Rumen fermentation
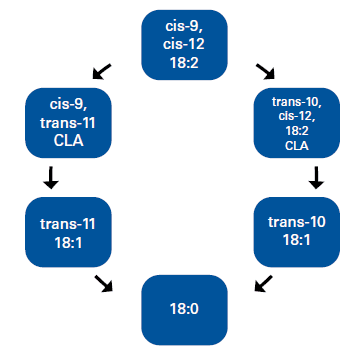
In the rumen fats suffer first hydrolysis and are later reduced to saturated fatty acids. The biohydrogenation (BH) of linoleic acid (cis-9, cis-12; 18:2) is produced in three reactions. First there is a isomerization that leads to synthesis of cis-9, trans- 11CLA), followed by two successive reductions, first to oleic (trans-11 C18:1) and then to stearic acid (C18:0; Harfoot and Hazelwood. 1988). But under certain altered rumen environmental conditions, BH can follow an alternate pathway which generates specific intermediaries, such as trans-10, cis-12 CLA and trans-10 18:1 (Bauman and Griinari.2001; Figure 1). These intermediaries can exit the prestomachs and the amount that will reach the small intestine for absorption depends on the rate of passage, the BH capacity of the rumen bacteria, and the concentration and profile of dietary PUFA that entered the rumen (Lock et al., 2006). Once absorbed they are carried through the circulation to the mammary gland where they cause a reduction in milk fat synthesis by interfering with the expression of genes that code lipogenic enzymes and key regulatory molecules. In conclusion, to produce MFD two conditions are necessary. One is the presence of PUFA in the rumen, the other, an altered rumen environment that causes their incomplete BH (Bauman and Griinari. 1998). Changes in the rumen environment are caused by the anti-bacterial action of PUFA, which penetrate the cell membrane of some bacterial species and cause cell damage by re-arrangement of cell membrane phospholipids (Jenkins, 2002). Due to the fact that the fatty acids which cause MFD are produced during BH of PUFA, one could assume that the amount of substrate present (linoleic and possibly linolenic acids) will determine the amount of intermediaries produced (Jenkins and Lock, 2008).
Figure 1. Bio-hydrogenation (BH) of linoleic acid Source: Adapted from Griinari and Bauman (1999)
Rumen bacteria ferment dietary carbohydrates and proteins and produce volatile fatty acids (VFA) as excretion products of their metabolism. Higher VFA concentrations in the rumen as a result of this fermentation lead to a drop in the pH which shifts microbial populations. These changes may cause a decrease in the digestion of structural carbohydrates and a reduction in the acetate to propionate ratio. In table 1 there´s a summary of the results obtained in 9 experiments comparing rations with different DG inclusions. Seven of these experiments also analyzed the concentration of VFA in the rumen. In five out of the seven relative concentration (moles/100 moles) of the fat-originating VFA acetic and butyric did not change significantly. In the experiments by Hippen et al. (2010) and Ranathunga et al. (2010) however, there was a reduction in the relative concentration of acetic acid in the rumen. In addition Hippen et al. (2010) reported a reduction in total VFA production in diets with 20% DDGS. The authors explained it because of possible reductions in effective fiber and the presence of PUFA. Ranathunga et al. (2010) proposed that the linear reduction observed in the relative acetate concentration observed at higher DDGS inclusions was probably due to similar reasons. This lower acetate concentration also resulted in a linear reduction in the acetate/propionate ratio. Both experiments indicated the rumen environment had been altered, with a typical example of MFD in the experiment by Hippen et al. (2010).
Table 1. Evaluation of the productive response in diets with different inclusion levels of DG.
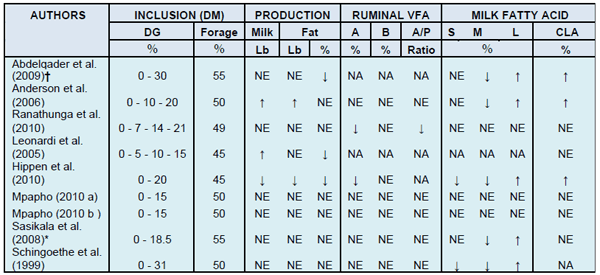
Abbreviations: DM (dry matter); VFA (Volatile fatty acids); FA (Fatty acids); DG (distillers grains); A (Acetate), B (Butyrate); P (Propionate); S (Short chain); M (Medium chain); L (long chain); CLA (t10-c 12 Conjugated linoleic acid); NS (not significant, P>0.05); NA (no data available), ↑ (increase); ↓ (decrease). †: The control diet has 16.3% (DM) of high protein DG (3.3% fat DM basis); *: In this trial the control diet was the one which had 10% corn distillers solubles.
Experimental diets
The NRC (2001) reports a fat content on a dry basis of 10% for traditional full-fat distillers grains, however, analysis published by the New York State Dairy One Lab (4819 samples, 2011), show an average fat content in DDGS of 12.6% (DM), and a range of values from 9.4 to 15.7%. These results show the high variability in the field of the fat content in DDGS. In this fat linoleic acid is the most abundant represents 53.7% of the total fatty acids (Moreau et al., 2011). In four research trials, where corn silage represented the most abundant forage in the ration, dietary linoleic acid increased as DG were increased in the diet (Figure 2). The total amount of linoleic acid in the diets without DG varied from 294 g (Schingoethe. 1999) to 439 g (Leonardi et al. 2005). The diet with the greatest content of linoleic acid was in the experiment by Leonardi et al. (2005) with 595 g and 15% DG on dry basis. Cows fed diets with corn silage as the sole or predominant source of forage seem to be more susceptible to MFD, particularly when supplementary unsaturated fats are added to the diet (Staples, 2006). Ruppert et al. (2003) showed that adding fat to diets where corn silage was the predominant forage resulted in MFD and this could be counteracted by substituting corn silage with alfalfa silage.
Figure 2. Percent increase of linoleic acid with the increase of DG in diets.
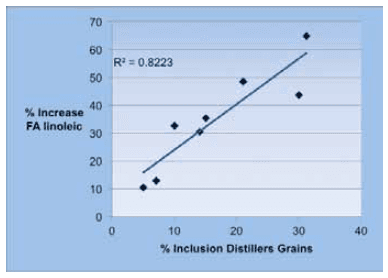
Source: Data was obtained from trials by: Abdelqader et al. (2009); Leonardi et al. (2005); Ranathunga et al. (2010) and Schingoethe et al. (1999).
Of the nine experiments in table 1 only Hippen et. al. (2010) reported a reduction in milk production, when comparing the control diet with one that contained 20% DDGS. Only two of the experiments showed milk production increases when DGGS were added to the diet. Leonardi et al. (2005) reported a linear increase in milk production as DDGS in the diet increased. Anderson et al. (2006) also observed greater production in diets with 10 and 20% DG both wet and dried. In this experiment no differences in milk production were observed with DG type (wet or dried) or inclusion level (10 or 20%). These results are in agreement with those published by Kalscheur et al. (2004) and Hippen et al. (2003) in which generally DG inclusion reduced milk production when in excess of 20% of the diet DM. The milk production increase observed by Leonardi and Anderson could be due to the rations with DG containing more total fat than the control. In the experiment by Hippen et al. (2010) however, the control diet included a source of inert fat with the objective of balancing its lipid content with that of the DDGS diet. No differences in milk production were observed in the remaining seven experiments.
Changes in milk fat
Milk fat is composed of a complex array of lipids 97% of them triglycerides (Christie, 1995). It is synthesized from fatty acids that come from the peripheral circulation (60%) or are synthesized de novo in the mammary gland (40%; Chilliard et al. 2000). Fatty acids with 4 to 14 carbons (C) and part of those with 16 C derive from de novo synthesis in the mammary gland with the main source of C being acetate and in less proportion beta-hydroxy-butyrate. The rest of the FA of 16 C and all of those with 18 C and longer chains derive from circulating FA, arising from the absorption of dietary lipids or fat mobilization from body reserves (Bauman and Griinari, 2001). During MFD there is reduced availability of most FA, but in addition the milk fat composition is modified particularly because this reduction affects mainly FA synthesized de novo (David and Brown, 1970). As a result the concentration in milk of short and medium chain FA is reduced, and the concentration of long chain FA increases (Bauman and Griinari, 2001).
An increase in BH intermediaries in milk fat such as trans-10, cis-12 CLA, is a sign of changes in the BH pathways of the PUFA in the rumen. Griinari et al. (1999) reported a strong correlation between the fat percent in milk and its content in trans-10, cis-12 CLA in cows fed a diet low in fiber and supplemented with sunflower oil. Moreover, the changes in the composition of milk FA during the abomasal infusion of trans-10, cis-12 CLA were consistent with those observed in diets that induce MFD (Bauman and Griinari, 2001). Only 3 out of 8 experiments presented in table 1 had an increase in trans-10, cis-12 CLA in milk in the diets that included DG. With the exception of the trial by Hippen et al. (2010) in which the increase in trans-10, cis-12 CLA was associated with the onset of MFD, in the trials by Abdelqader et al.(2009), and Anderson et al., (2006) the increase in this isomer was not accompanied by a reduction in milk fat production. Although both studies report statistically significant differences, the low concentration at which this CLA occurs may be insufficient to result in MFD.
On occasions dairy cow diets can increase milk production with no changes in fat yield which results in reduced milk fat concentration. This does not constitute MFD because fat synthesis has not been affected as the concentration drop results from dilution. In one third of the trials described in table 1 milk fat concentration was reduced. Abdelqader et al. (2009 ) and Leonardi et al. (2005) reported a decrease in milk fat percent and no differences in fat yield. Conversely, in the experiment by Hippen et al. (2006), there was a decrease both in fat percent and yield. These latter results agree with those published by Kalscheur (2005), where in a meta-analysis of several published trials DG led to MFD only when diets had less than 50% forage or less than 22% of forage NDF. In the trial by Hippen et al. (2010), the diet had 45% forage and 19.3% forage NDF which lead to MFD. In their trial the feed retained in the first two screens of the 3-sieve Penn State Particle Separator was similar, although in the diet with DDGS there was approximately 3% less and 3.8% more in the third screen and bottom pan, respectively. In spite of all fractions being within suggested levels for high producing dairy cows rations (Zebeli et al. 2007), the fact that some material from the third tray passed to the bottom pan represents a higher content of easily fermentable nutrients and potentially also greater rate of passage, which has been associated with reduced fat concentration and yield (Grant et al., 1990; Beauchemin et al., 1997; Kononoff et al., 2003; Soita et al., 2005). Similar results between TMR particle size and milk fat% were reported by Nydam et al.(2008) on an epidemiological study to establish risk factors that contribute to MFD in commercial dairy farms feeding Rumensin®. Particle separation of TMR samples into using the 3-box, 2-sieve Penn State Particle Separator gave significant relationships between milk fat % and particle size that were positive for the middle screen (P =0.008) and negative for the bottom pan (P=0.003). Milk fat % did not relate to TMR particle size on the top screen. When all TMR variables (e.g. TMR variables such as DM, starch, NDF, etc.) and herd management factors were analyzed through multi-variate regression and step-wise regression elimination of non-significant variables, the factors that remained in the model as significant were the DM concentration of the TMR and percentage of particles remaining on the bottom pan of the 2-sieve Penn State Particle Separator. Both these factors were responsible for 21% of the variation observed in milk fat % in their experiment. In the trial by Anderson et al. (2006) reported in table 1 there was an increase in fat production together with an increase in milk yield. The other 6 experiments addressed did not show effects in either concentration or fat when DG were added to the diets.
Adding DG to the diet reduced the relative proportion (g/100 g of FA) of medium chain FA, and increased the concentration of long chain FA in 4 of the 8 trials analyzed in table 1. Moreover, in two of them, there´s also a reduction in short chain FA. Possible explanations for this change:
• Reduced de novo synthesis of FA due to inhibitory effect of intermediaries of BH on lipogenic enzymes in the mammary gland (Bauman and Griinari. 2001);
• More circulating long-chain FA arriving to the mamary gland (Chilliard et al. 2000);
• Reduced acetate and beta-hydroxy-butyrate availability to synthesize FA in the mammary gland (David and Brown, 1970).
Conclusions
Some practical recommendations can be drawn from the results of these experiments in order to maximize distillers grains inclusion without significant negative effects on milk fat yield or concentration.
1. Although formulating diets for adequate NDF concentrations is important, it needs to be accompanied by particle size assessment of the ration in cows fed TMR.
2. Although adding buffers to the diet (e.g. bicarbonate) can ameliorate the effects of low rumen pH their effect is transitory and is not a substitute for adequate particle sized rations.
3. The amounts and fat composition of other feedstuffs included in the diet will dictate how much distillers grains can be safely included.
4. Even with adequate particle size avoid the inclusion of more than 10 percent wet DG products when fermented corn feedstuffs (e.g corn silage, HMC, etc.) constitute already 50% or more of the diet dry matter.
References
Abdelqader, M. M., A. R. Hippen, K. F. Kalscheur, D. J. Schingoethe, and A. D. Garcia. 2009. Isolipidic additions of fat from corn germ, corn distillers grains, or corn oil in dair y cow diets. Journal of Dairy Science 92: 5523–5533.
Anderson, J. L., D. J. Schingoethe, K. F. Kalscheur, and A. R. Hippen. 2006. Evaluation of dried and wet distillers grains included at two concentrations in the diets of lactating dair y cows. Journal of Dair y Science 89: 3133–3142.
Bauman, D.E., J. M. Griinari. 2001. Regulation and nutritional manipulation of milk fat: low-fat milk syndrome. Livest. Prod. Sci., 70:15–29.
Beauchemin, K. A., L. M. Rode, and M. J. Eliason. 1997. Chewing activities and milk production of dair y cows fed alfalfa as hay, silage, or dried cubes of hay or silage. J. Dair y Sci. 80:324–333.
Cyriac, J., M. M. Abdelqader, K. F. Kalscheur, A. R. Hippen, and D. J. Schingoethe. 2005. Effect of replacing forage fiber with nonforage fiber in lactating dair y cow diets. J. Dair y Sci. 88(Suppl.1):252. (Abstr.)
Chilliard, Y., A. Ferlay, R. M. Mansbridge and M. Doreau. 2000. Ruminant milk fat plasticity: nutritional control of saturated, polyunsaturated, trans and conjugated fatty acids. Ann. Zootech. 49:181–205.
Christie W. W. Composition and structure of milk lipids. 1995. In: Advanced Dairy Chemistry, Vol. 2: Lipids. Eds. P. F. Fox, Chapman and Hall, London (UK), pp. 1–36.
Dairy One Forage Lab. 1Valores acumulados desde el 01/05/2000 hasta el 30/04/2011. Disponible online: http://www.dairyone.com . Acceso noviembre 2011.
Davis, C. L., Brown, R. E. 1970. Low-fat milk syndrome. In Physiology of Digestion and Metabolism in the Ruminant, ed. AT Phillipson, pp. 545–65. Newcastle upon Tyne, UK: Oriel
Díaz-Royón, F. and A. García. 2012. Características de los lípidos en los granos de destilería. Albeitar. 152:28-30.
Grant, R. J., V. F. Colenbrander, and D. R. Mertens. 1990. Milk fat depression in dair y cows: Role of particle size of alfalfa hay. J. Dair y Sci. 73:1823–1833.
Griinari, J. M., Nurmela, K., Dwyer, D. A., Barbano, D. M., Bauman, D. E. 1999. Variation of milk fat concentration of conjugated linoleic acid and milk fat percentage is associated with a change in ruminal biohydrogenation. J. Anim. Sci. 77(Suppl.1):117–18 (Abstr.)
Harfoot C.G. and Hazlewood GP. 1988. Lipid metabolism in the rumen. In The Rumen Microbial Ecosystem, ed. PN Hobson, pp. 285– 322. Amsterdam: Elsevier
Hippen, A. R., K. N. Linke, K. F. Kalscheur, D. J. Schingoethe, and A. D. Garcia. 2003. Increased concentrations of wet corn distillers grains in dair y cow diets. Journal of Dair y Science 86(Suppl. 1): 340. (Abstr.)
Hippen, A. R., D. J. Schingoethe, K. F. Kalscheur, P. Linke, D. R. Rennich, M. M. Abdelqader, and I. Yoon. 2010. Saccharomyces cerevisiae fermentation product in dair y cow diets containing dried distillers grains plus solubles. Journal of Dairy Science 93: 2661– 2669.
Jenkins, T. C. 2002. Lipid transformations by the rumen microbial ecosystem and their impact on fermentative capacity. pp 103-117 in Gastrointestinal Microbiology in Animals, S. A. Martin (Ed.), Research Signpost, Kerala, India.
Jenkins, T. C and A. L. Lock. 2008. Fat Metabolism in the Rumen and Effect of Distillers Grains on Milk Components. http://www.ahdairy.com/our-products/msds-info-sheets.aspx?MainMenuSelection=our_products&MenuSelection=bypass_ fats&brand=2&category=4&type=0
Kalscheur, K. F., A. L. Justin, A. R. Hippen, and D. J. Schingoethe. 2004. Increasing wet distillers grains in diets of dair y cows on milk production and nutrient utilization. J. Dair y Sci. 87(Suppl.1):465. (Abstr.)
Kalscheur, K. F. 2005. Impact of feeding distillers grains on milk fat, protein, and yield. Proceedings Distillers Grains Technology Council, 9th Annual Symposium, Louisville, KY
Kennelly J.J. 1996, The fatty acid composition of milk fat as influenced by feeding oilseeds, Anim. Feed Sci. Tech. 60:137–152.
Kononoff, P. J., A. J. Heinrichs, and D. R. Buckmaster. 2003. Modification of the Penn State forage and total mixed ration particle separator and the effects on moisture content on its measurements . J. Dair y Sci. 86:1858–1863.
Leonardi, C., S. Bertics, and L. E. Armentano. 2005. Effect of increasing oil from distillers grains or corn oil on lactation performance. Journal of Dairy Science 88: 2820–2827.
Lock A. L., T. R. Overton, K. J. Harvatine, J. Giesy, and D. L. Bauman. 2006. Milk fat depression: Impact of dietary components and their interaction during rumen fermentation. Proc. Cornell Nutr. Conf. pp. 75-85.
Moureau, R. A., K. Liu., J. K. Winkler-Moser, V. Singh. 2011. Changes in Lipid Composition During Dr y Grind Ethanol Processing of Corn. J. Am. Oil Chem. Soc., 88:435–442.
National Research Council. 2001. Nutrient Requirements for Dair y Cattle. 7th rev. ed. Natl. Acad. Sci., Washington, DC, USA.
Nydam, D. V., T. R. Overton, G. D. Mechor, D. E. Bauman, and T. C. Jenkins. 2008. Risk factors for bulk tank milk fat depression in northeast and Midwest US dair y herds feeding monensin. American Association of Bovine Practioners, Charlotte, NC.
Ranathunga, S. D., K. F. Kalscheur, A. R. Hippen, and D. J. Schingoethe. 2010. Replacement of starch from corn with non-forage fiber from distillers grains and soy hulls in diets of lactating dair y cows. Journal of Dair y Science 93: 1086–1097.
Ruppert, L. D., J. K. Drackley, D. R. Bremmer, and J. H. Clark. 2003. Effects of tallow in diets based on corn silage or alfalfa silage on digestion and nutrient use by lactating dair y cows. J. Dair y Sci. 86:593-609.
Sasikala-Appukuttan, A. K., D. J. Schingoethe, A. R. Hippen, K. F. Kalscheur, K. Karges, and M. L. Gibson. 2008. The feeding value of corn distillers solubles for lactating dair y cows. Journal of Dairy Science 91: 279–287.
Schingoethe, D. J., M. J. Brouk, and C. P. Birkelo. 1999. Milk production and composition from cows fed wet corn distillers grains. J. Dair y Sci. 82:574–580.
Schingoethe, D. J., K. F. Kalscheur , A. R. Hippen , and A. D. Garcia. 2009. Invited review: The use of distillers products in dairy cattle diets. J. Dairy Sci. 92:5802–5813.
Soita, H. W., M. Fehr, D. A. Christensen, and T. Mutsvangwa. 2005. Effects of corn silage particle length and forage:concentrate ratio on milk fatty acid composition in dair y cows fed supplemental flaxseed. J. Dair y Sci. 88:2813–2819.
Staples, C. R. 2006. Milk fat depression in dair y cows - Influence of supplemental fats. Florida Ruminant Nutrition Symposium, Gainesville, Florida.
Zebeli, Q., J. Dijkstra, M. Tafaj, H. Steingass, B. N. Ametaj, and W. Drochner. 2007. Modeling the adequacy of dietar y fiber in dairy cows based on the responses of ruminal pH and milk fat production to composition of the diet. J. Dairy Sci. 91:2046–2066.
Related topics
Authors:
South Dakota State University
South Dakota State University
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.







.jpg&w=3840&q=75)