Comparison between three diagnostic tests to detect abortion caused by infectious bovine rhinotracheitis in dairy herds
Published: September 4, 2006
By: Alfa Bracho Cárdenas - Carlos J. Jaramillo Arango - José Juan Martínez Maya - Juan Antonio Montaño Hirose - Arturo Olguín y Bernal
A viral agent associated to outbreaks of respiratory diseases of bovines, is the Herpes bovis 1 (BHV-1) of the family Herpesviridae which provokes the infectious bovine rhinotracheitis (IBR). According to the genomic and antigenic type, the BHV-1 divides in BHV-1.1 and BHV-1.2, which, consequently, subdivides in BHV-1.2a and BHV-1.2b.1 The clinical manifestations and give way of the disease depend on the anatomical site of the infection, age, and immunological condition of the host,2,3 provoking from a mere respiratory profile (rhinotracheitis, conjunctivitis), genital (vaginitis, epididymitis, orchitis and abortion), enteritis, to a systemic disease.1,2
The disease has an averageincubation period of 21 days.4 When the disease occurs in a subclinical presentation,1it is manifested by the interruption of gestation, which is seen in 25% to 50%of pregnant cows; if the infection is early manifested during the gestation periodit can provoke embrionary reabsorption, but if the death occurs during the firstquarters of pregnancy (when this is dependent on the corpus luteum byprogesterone) the interval between fetal death, luteolysis and expulsion is enoughfor the fetal autolysis. Generally, abortion occurs during the third quarterof pregnancy, in a period of time no longer than three weeks in which the fetusis autolyzed.1,2,5.6
The prevalence in herdsdepends on factors as: the immune state of the dam, the period of gestation inwhich the infection occurs or if it manifests itself, the tropism and virulenceof the agent.1
In samples send to theNational Center of Diagnostic Services in Animal Health, between January 1992to February 1996, a frequency of 56.53% positives was obtained in 18 states ofthe Mexican Republic.
The transmission of thevirus is carried out through the respiratory secretions, ocular or reproductivesystem such as: semen, embryo implants and obstetric procedures.7
The virus can be latent preserved in herds, by the presence of carrier bovinesof field strains that are occasionally reactivated by diverse stimuli withthe consequent viral replication through the respiratory and reproductive tracts,which favors the transmission to susceptible animals. This situation makes IBR a diseaseof great diffusion and very difficult control.5,7,8
From an economical pointof view, the importance of IBR has not been completely evaluated, butit is calculated that in ten years 18% of the herd is removed by infectious diseases,from which abortion is relevant.9 The IBR is an enzootic disease with obligatorynotification in Mexico, due to its significant effect in livestock production. Besides,it belongs to group B of the International Zoosanitary Code for its strategicimportance for the health animal actions in each country.4, 10
In México, the IBR is an infectious disease of great importance for dairyherds, since in the majority of the animals, the disease goes through a subclinicalform; it has as principle characteristic the abortion and consequently the lossof the product and milk production, affecting the reproductive and productiveparameters and increasing the economic losses.
The common techniquesfor the IBR diagnosis are: seroneutralization, compliment fixation, ELISA, viralneutralization, viral isolation, immunofluorescence and immunohistochemistry.1,11
The seroneutralization test has the purpose to search for neutralized antibodiesin the animals´ serum. If the conventional incubation of virus is usedduring 1 hour at 37°C, the test presents an 89.2% of sensibility and a nearspecificity of 100%. Nevertheless, if the reactors are incubated during 24 h,it is possible to increase the sensibility up to 94.4%, but the specificity decreasesto 93.2%. A limit to this test is that it is only certain in non vaccinated herds.1
The immunofluorescence test is used for the identification of antigens or antibodiesin fresh material (kidney and adrenal glands).1,13-15 The indirect test is moresensible, but not enough to justify its use, because it is slower.15,16 In thedirect exam of frozen sections of fetal kidney, the test has superior specificityof 90% and a sensibility of 67%.15,16
For the immonoperoxidase technique impressions of suspect tissue weremade on slides. This last ones are incubated with antibodies against theIBR virus, combined with an enzyme , generally radish peroxidase. Afterbeing rinsed, the impression is treated with the substrate of the enzyme anda chromgene which products provoke a colored reaction directly proportional tothe quantity of antigen present in the sample.17,18 According to Smith etal.,17 this test has a sensibility of 94%.
This technique has two advantages for the diagnosis; the first one does not needequipped microscopes for the observation with ultraviolet light, and second, theinfected cellular cultures can be fixed directly in the same microplatesutilized for the viral isolation.
Viral isolation is anotherdiagnostic test that has the inconvenient of its slowness, sinceit delays at least a week; it requires equipment, material and specialized personnel.If the samples are not immediately processed, they should be frozen at –70° C.Cultures of monolayers, homologous or primary cells are incubated at 37° Cand are daily observed to compare the apparition of the cytopathic effect, thiseffect must be valued in comparison with non-inoculated cultures called negativecontrols, especially in the case of viruses that need incubation periods longerthan a week.10,19,20
Some authors mentionthat the abortion diagnosis is only confirmed by fetal tissue examination.1 Althoughthe different viral isolations obtained from diverse clinical presentations ofthe disease differ in its affinity for different organs, result serologicallyidentical.16
Officially, the IBR diagnosis must utilize the seroneutralization test, whichhas been internationally accepted as reference test.10,20
As consequence of thelast mentioned, it is necessary to count with an alternative test of equal valueor better specificity, sensibility and faster, in order to appropriately detectinfected animals and fetuses in the existing field conditions.
Material and methods
The study is carried out in the Agricultural and Industrial Complex of Tizayuca(CAIT), Tizayuca, Hidalgo, Mexico, by means of a convenient non-probabilisticsampling in which the aborted fetuses were evaluated and obtained from the notificationof employees or owners, or both, of the stables to the laboratory of pathologyof CAIT, as well as serum of the progenitor cows of the fetuses through the studyperiod.
The obtained fetuses were subjected to necropsy as described by Aluja,21 dissectingthe following: liver, spleen, kidney and lungs, obtaining approximately 2 cm3of each one, placing them in labeled sterile glass jars and maintaining themfrozen at –4°C, during one or two days, until their arrival to theNational Center of Diagnostic Services in Animal Health (CENASA), where theywere analized.
The day in which eachcow had an abortion, 10 mL of blood were extracted from the coccygeal vein. Theserum was clarified by centrifugation at 1,875 gduring 15 minutes andwas deposited in sterile recipients; this process was repeated 30 and 60 daysafter the abortion. The samples were frozen conserved at –4°C untiltheir analysis.
Organ analysis
Each organ sample was subjected to tests: viral isolation (VI), immunoperoxidaseon plate (IPP), direct immunoperoxidase (IP) and direct immunofluorescence (IF).
For the diagnosis by VI a maceration was performed with approximately 0.5 g of each organ (2 g total) in 18 mL of MEM (minimal essential medium), containing 0.2 mL of bicarbonate at 2%. It was filtered through a membrane of 0.45 μm, the technique wasperformed as the World Organization Animal Health (OIE).18,19
MDBK and PK15 cellular lines were used, inoculating five bottles of 5 mL foreach cellular line and sample; the bottles were daily observed during five daysin order to detect cytophatic effect. If there was non effect during those days,three to five passes for each fetus sample was performed; simultaneously, IPPon plate of 96 wells was done to put in evidence the IBR viral isolation.
The IP and IF techniques were carried out as recommended by the OIE and Foodand Agriculture Organization (FAO),19,20, which basically consisted in cuttingand placing an organ sample (liver, spleen, kidney or lung) of approximately1 cm2 with gelatin and freezing it in a cryostat to perform three cutsof 3-5 μm of thickness, placing them on a slide, and fixing them with acetoneat 30%, freezing them during 10 minutes.
Immunofluorescence
Each slide was washed with PBS, decanted and dried, then 25μL of conjugateagainst IBR was added and incubated at 37°C during 30 minutes in humid chamber,later on they were washed with PBS and dried in order to mount them with bufferedglicerine to be observed at the epifluorescence microscope.
Immunoperoxidase
Each slide was washed with SL solution (PBS and Tween), decanted and driedin order to add conjugate (reference serum) and be incubated for an hour at 37°Cin humid chamber in a CO2 stove; later on, they were decanted and washed threetimes with SL solution, then dried and conjugated G protein was added, they wereone more time incubated for 30 minutes at 37°C in humid chamber in a CO2stove, and were washed three times with SL solution.
Fifty μL of 3,9 aminoethylcarbazolwere added, as diluted indicator in sodium phosphate and citric acid solutions,plus the substrate and incubated for 20 minutes at room temperature (the indicatorwas prepared at the moment of usage); it was washed and observed in optic microscopewith dry low, dry high and immersion objective.
For both tests, a positive sample was considered, when at least one of the fourorgan samples of only one fetus gave positive reaction to IP and to direct IF.
Serum analysis
For the dams’ serum, seroneutralization test was performed (SN) withinthe established by the OIE and the Food Administration Organization (FAO).18,19
Statistic analysisBy comparing direct IP and IF with VI the proportions of: true positives, truenegatives, false positives, false negatives, Sp, Se, +PV and –PV, wereobtained in each test. With these results the tests of IP and IF were compared,and the gross concordance index (GCI), and the concordance by Kappa index (KI)with SN, were calculated.23,24 To carry out the statistic analysis, anEPIINFO 6 program was used.
EVALUATION OF 49 SAMPLES OF ABORTED FETUSES BY THE IMMUNOFLUORESCENCE, IMMUNOPEROXIDASEAND VIRAL ISOLATION TESTS FOR IBR, CAIT; MEXICO, 1997
The disease has an averageincubation period of 21 days.4 When the disease occurs in a subclinical presentation,1it is manifested by the interruption of gestation, which is seen in 25% to 50%of pregnant cows; if the infection is early manifested during the gestation periodit can provoke embrionary reabsorption, but if the death occurs during the firstquarters of pregnancy (when this is dependent on the corpus luteum byprogesterone) the interval between fetal death, luteolysis and expulsion is enoughfor the fetal autolysis. Generally, abortion occurs during the third quarterof pregnancy, in a period of time no longer than three weeks in which the fetusis autolyzed.1,2,5.6
The prevalence in herdsdepends on factors as: the immune state of the dam, the period of gestation inwhich the infection occurs or if it manifests itself, the tropism and virulenceof the agent.1
In samples send to theNational Center of Diagnostic Services in Animal Health, between January 1992to February 1996, a frequency of 56.53% positives was obtained in 18 states ofthe Mexican Republic.
The transmission of thevirus is carried out through the respiratory secretions, ocular or reproductivesystem such as: semen, embryo implants and obstetric procedures.7
The virus can be latent preserved in herds, by the presence of carrier bovinesof field strains that are occasionally reactivated by diverse stimuli withthe consequent viral replication through the respiratory and reproductive tracts,which favors the transmission to susceptible animals. This situation makes IBR a diseaseof great diffusion and very difficult control.5,7,8
From an economical pointof view, the importance of IBR has not been completely evaluated, butit is calculated that in ten years 18% of the herd is removed by infectious diseases,from which abortion is relevant.9 The IBR is an enzootic disease with obligatorynotification in Mexico, due to its significant effect in livestock production. Besides,it belongs to group B of the International Zoosanitary Code for its strategicimportance for the health animal actions in each country.4, 10
In México, the IBR is an infectious disease of great importance for dairyherds, since in the majority of the animals, the disease goes through a subclinicalform; it has as principle characteristic the abortion and consequently the lossof the product and milk production, affecting the reproductive and productiveparameters and increasing the economic losses.
The common techniquesfor the IBR diagnosis are: seroneutralization, compliment fixation, ELISA, viralneutralization, viral isolation, immunofluorescence and immunohistochemistry.1,11
The seroneutralization test has the purpose to search for neutralized antibodiesin the animals´ serum. If the conventional incubation of virus is usedduring 1 hour at 37°C, the test presents an 89.2% of sensibility and a nearspecificity of 100%. Nevertheless, if the reactors are incubated during 24 h,it is possible to increase the sensibility up to 94.4%, but the specificity decreasesto 93.2%. A limit to this test is that it is only certain in non vaccinated herds.1
The immunofluorescence test is used for the identification of antigens or antibodiesin fresh material (kidney and adrenal glands).1,13-15 The indirect test is moresensible, but not enough to justify its use, because it is slower.15,16 In thedirect exam of frozen sections of fetal kidney, the test has superior specificityof 90% and a sensibility of 67%.15,16
For the immonoperoxidase technique impressions of suspect tissue weremade on slides. This last ones are incubated with antibodies against theIBR virus, combined with an enzyme , generally radish peroxidase. Afterbeing rinsed, the impression is treated with the substrate of the enzyme anda chromgene which products provoke a colored reaction directly proportional tothe quantity of antigen present in the sample.17,18 According to Smith etal.,17 this test has a sensibility of 94%.
This technique has two advantages for the diagnosis; the first one does not needequipped microscopes for the observation with ultraviolet light, and second, theinfected cellular cultures can be fixed directly in the same microplatesutilized for the viral isolation.
Viral isolation is anotherdiagnostic test that has the inconvenient of its slowness, sinceit delays at least a week; it requires equipment, material and specialized personnel.If the samples are not immediately processed, they should be frozen at –70° C.Cultures of monolayers, homologous or primary cells are incubated at 37° Cand are daily observed to compare the apparition of the cytopathic effect, thiseffect must be valued in comparison with non-inoculated cultures called negativecontrols, especially in the case of viruses that need incubation periods longerthan a week.10,19,20
Some authors mentionthat the abortion diagnosis is only confirmed by fetal tissue examination.1 Althoughthe different viral isolations obtained from diverse clinical presentations ofthe disease differ in its affinity for different organs, result serologicallyidentical.16
Officially, the IBR diagnosis must utilize the seroneutralization test, whichhas been internationally accepted as reference test.10,20
As consequence of thelast mentioned, it is necessary to count with an alternative test of equal valueor better specificity, sensibility and faster, in order to appropriately detectinfected animals and fetuses in the existing field conditions.
Material and methods
The study is carried out in the Agricultural and Industrial Complex of Tizayuca(CAIT), Tizayuca, Hidalgo, Mexico, by means of a convenient non-probabilisticsampling in which the aborted fetuses were evaluated and obtained from the notificationof employees or owners, or both, of the stables to the laboratory of pathologyof CAIT, as well as serum of the progenitor cows of the fetuses through the studyperiod.
The obtained fetuses were subjected to necropsy as described by Aluja,21 dissectingthe following: liver, spleen, kidney and lungs, obtaining approximately 2 cm3of each one, placing them in labeled sterile glass jars and maintaining themfrozen at –4°C, during one or two days, until their arrival to theNational Center of Diagnostic Services in Animal Health (CENASA), where theywere analized.
The day in which eachcow had an abortion, 10 mL of blood were extracted from the coccygeal vein. Theserum was clarified by centrifugation at 1,875 gduring 15 minutes andwas deposited in sterile recipients; this process was repeated 30 and 60 daysafter the abortion. The samples were frozen conserved at –4°C untiltheir analysis.
Organ analysis
Each organ sample was subjected to tests: viral isolation (VI), immunoperoxidaseon plate (IPP), direct immunoperoxidase (IP) and direct immunofluorescence (IF).
For the diagnosis by VI a maceration was performed with approximately 0.5 g of each organ (2 g total) in 18 mL of MEM (minimal essential medium), containing 0.2 mL of bicarbonate at 2%. It was filtered through a membrane of 0.45 μm, the technique wasperformed as the World Organization Animal Health (OIE).18,19
MDBK and PK15 cellular lines were used, inoculating five bottles of 5 mL foreach cellular line and sample; the bottles were daily observed during five daysin order to detect cytophatic effect. If there was non effect during those days,three to five passes for each fetus sample was performed; simultaneously, IPPon plate of 96 wells was done to put in evidence the IBR viral isolation.
The IP and IF techniques were carried out as recommended by the OIE and Foodand Agriculture Organization (FAO),19,20, which basically consisted in cuttingand placing an organ sample (liver, spleen, kidney or lung) of approximately1 cm2 with gelatin and freezing it in a cryostat to perform three cutsof 3-5 μm of thickness, placing them on a slide, and fixing them with acetoneat 30%, freezing them during 10 minutes.
Immunofluorescence
Each slide was washed with PBS, decanted and dried, then 25μL of conjugateagainst IBR was added and incubated at 37°C during 30 minutes in humid chamber,later on they were washed with PBS and dried in order to mount them with bufferedglicerine to be observed at the epifluorescence microscope.
Immunoperoxidase
Each slide was washed with SL solution (PBS and Tween), decanted and driedin order to add conjugate (reference serum) and be incubated for an hour at 37°Cin humid chamber in a CO2 stove; later on, they were decanted and washed threetimes with SL solution, then dried and conjugated G protein was added, they wereone more time incubated for 30 minutes at 37°C in humid chamber in a CO2stove, and were washed three times with SL solution.
Fifty μL of 3,9 aminoethylcarbazolwere added, as diluted indicator in sodium phosphate and citric acid solutions,plus the substrate and incubated for 20 minutes at room temperature (the indicatorwas prepared at the moment of usage); it was washed and observed in optic microscopewith dry low, dry high and immersion objective.
For both tests, a positive sample was considered, when at least one of the fourorgan samples of only one fetus gave positive reaction to IP and to direct IF.
Serum analysis
For the dams’ serum, seroneutralization test was performed (SN) withinthe established by the OIE and the Food Administration Organization (FAO).18,19
Statistic analysisBy comparing direct IP and IF with VI the proportions of: true positives, truenegatives, false positives, false negatives, Sp, Se, +PV and –PV, wereobtained in each test. With these results the tests of IP and IF were compared,and the gross concordance index (GCI), and the concordance by Kappa index (KI)with SN, were calculated.23,24 To carry out the statistic analysis, anEPIINFO 6 program was used.
Results
During the study period, 86 samples of aborted fetuses were obtained; from these, only 49 were possible to analyze due to autolysis.
During the study period, 86 samples of aborted fetuses were obtained; from these, only 49 were possible to analyze due to autolysis.
Viral isolation
From the 49 analyzed fetus samples, in 29 (59%) the IBR virus was isolated (Table 1).
From the 49 analyzed fetus samples, in 29 (59%) the IBR virus was isolated (Table 1).
Immunofluorescence and immunoperoxidase
The 26.5% of the samples were positive to IF and 89.7% to IP (Table 1). While analyzing each test per organ, it was found that for IF the positive frequency varied from 13% to 18% in spleen and liver, respectively, without finding significant difference ( p= 0.9205) (Table 2). For IP by organ it was found a positive variation of 42% to 74% in liver and kidney, respectively. Such variation was significant (p= 0.012) (Table 2).
The 26.5% of the samples were positive to IF and 89.7% to IP (Table 1). While analyzing each test per organ, it was found that for IF the positive frequency varied from 13% to 18% in spleen and liver, respectively, without finding significant difference ( p= 0.9205) (Table 2). For IP by organ it was found a positive variation of 42% to 74% in liver and kidney, respectively. Such variation was significant (p= 0.012) (Table 2).
Sensibility (Se), specificity (Sp) and concordance between viral isolation and inmunofluorescence
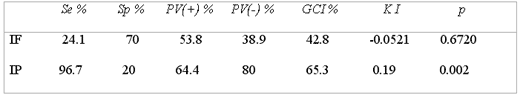
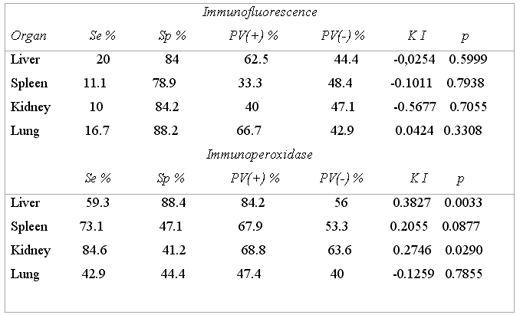
The IF test presented Se of 24.1% and Sp of 70%, +PV of 53.8% and –PV of 38.9%; the concordance for KI between both tests was of –0.052 , which was not significant (KI = -0.052, p= 0.6) (Table 3), Per organ , IF presented very low Se since it goes from 10% to 20%, meanwhile Sp goes from 79 to 88% for spleen and lung, respectively; in all organs the concordance for KI with VI was not significant (Table 4).
The IF test presented Se of 24.1% and Sp of 70%, +PV of 53.8% and –PV of 38.9%; the concordance for KI between both tests was of –0.052 , which was not significant (KI = -0.052, p= 0.6) (Table 3), Per organ , IF presented very low Se since it goes from 10% to 20%, meanwhile Sp goes from 79 to 88% for spleen and lung, respectively; in all organs the concordance for KI with VI was not significant (Table 4).
Sensibility, specificity and concordance between viral isolation and inmunofluorecence
The IP test presented Se of 96.7% and Sp of 20%, a +PV of 64.4% and a –PV of 80%. The concordance for KI between both tests was 0.19, which was not significant (KI =0.19, p=0.002) (Table 3). By organ: IP presents variations in Se, Sp, +PV and –PV, obtaining the best response in liver with a significant concordance (KI = 0.3827, p = 0.0033) (Table4).
The IP test presented Se of 96.7% and Sp of 20%, a +PV of 64.4% and a –PV of 80%. The concordance for KI between both tests was 0.19, which was not significant (KI =0.19, p=0.002) (Table 3). By organ: IP presents variations in Se, Sp, +PV and –PV, obtaining the best response in liver with a significant concordance (KI = 0.3827, p = 0.0033) (Table4).
Seroneutralization
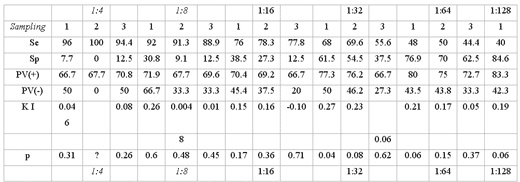
Of the 49 analyzed fetuses it was only possible to obtain serum samples from 38 of their dams in the first sampling, 34 in the second and 26 in the third, since the herd owners did not allow the obtainment of more samples. The average value of antibodies against IBR in the animals that had an abortion was of 1:32, in general. When comparing SN to VI, variations in Se and Sp were found determined by different cutting spots. The results showed significant concordance by only considering as positives the serums of the first sampling with values of 1:32 or greater (KI = 0.27, p = 0.04), and even in this case it shows that Se was of 68% and Sp of 61.5% (Table5).
Of the 49 analyzed fetuses it was only possible to obtain serum samples from 38 of their dams in the first sampling, 34 in the second and 26 in the third, since the herd owners did not allow the obtainment of more samples. The average value of antibodies against IBR in the animals that had an abortion was of 1:32, in general. When comparing SN to VI, variations in Se and Sp were found determined by different cutting spots. The results showed significant concordance by only considering as positives the serums of the first sampling with values of 1:32 or greater (KI = 0.27, p = 0.04), and even in this case it shows that Se was of 68% and Sp of 61.5% (Table5).
Discussion
In relation with IP characteristics as diagnostic test, it was found that Se was greater (96.7%) to the one found by Smith et al. and Delgado et al.,15,17 who determined a Se of 96% for avidin-biotin and 83.3% by direct technique, respectively. Nevertheless, the Sp found (20%) was less than the notified by Delgado et al.,15 which was nearer to 100%. The high quantity of positive false derived from the low specificity, was probably due to a focal unspecific coloration that the sample proportionates and gives the morphology of the blood vessels and the presence of red blood cells in this, as it has been mentioned by Smith12 and Theodoris et al.25 Another possible explanation is that the serum or the sample, or both, were contaminated with other type of proteins, such as the bacteriological origin and to the possible cross reaction with other herpes viruses.26 Another cause in the variation of the results could be due to a low concentration of serum antibodies that is used to reveal the presence of the antigen, as Kramps mentions.3 Furthermore, the coloration can not be visible if the concentration in the tissue is minimal, impeding that the conjugate detects its presence, as mentioned by Delgado and Kelling.15 26
The IP performed in plate to confirm the VI favors the possibility to find theantigen, since, for the elaboration of this technique, it is necessary to performa maceration of all the organs (pool), while the direct technique of the frozencut does not guarantee, in the evaluated tissue portion, that the antigen isin seropositive animals. Posposil et al 27 have demonstrated the presenceof the virus in liver, kidney, lung and other aborted fetus organs of seronegativeanimals and in some experimentally infected seropositives. In sever cases ofautolysis , the samples could not be evaluated by IP neither for IF, becausethe tissue detached itself at the moment of washing during the techniqueprocess.
In the cuts of frozentissue, the anatomy of the evaluated organ was not always seen due to the autolysis,this condition did not interfere with the interpretation of the IP test, becausethe obtained coloration in the positive cases was evident; therefore, all thetissues could be evaluated, not so with IF in which, in autolysis conditions,unspecific redish coloration was observed, difficulting the interpretation ofthe results, which coincides with Theodoris et al and Reed etal.25,28. It is to be mention that the samples that did not presented severeautolysis, the encounter fluorescence was focal and dim uniformly distributedin the nucleus and cytoplasm, specifically in kidney, which coincides with thefinding by Reed et al.28
The Se and Sp obtained by IF (24.1%, 70%) were lower to ones found by Delgado etal and Smith et al 15,17 whom obtained Se of 67% and 83.3%, respectively,not so with Sp, since Delgado et al 15 found 100% under laboratory conditions.The lower Se could be due to the autolysis of the samples, as well as the samereasons described for IP and the treatment given to the sample, which can affectthe antigenic site and the quality of the antibodies in the serum, in order toreveal the presence of the antigen.26
The correlation between the VI and IF found by Reed et al 28 was of 50%. Onthe other hand, Bratanich et al 14 mention a concordanceof 83%, which is higher than the one found in the present study of only 42.9%.
In this study the organsof election for the samples send to the laboratory were: liver, kidneyand lung, which coincide with the indicated by Smith et al 17
The variation in the values of Sn, which go from 1:2 to higher than 1:128, incows at the moment of abortion, coincide with the indicated by S. Van Drunen etal. 29 A problem with the serological evaluation was trying to differentiatethe positive serums, since antibodies induced by natural infection or vaccination couldbe found, most of all if it is considered that the titles can be similar to vaccinationas to natural infection.30 It is important to point out that in CAIT, atthe beginning of the sampling, some animals were inoculated with thermosensibleattenuated vaccine and during the study every cow received inactivated or modifiedvaccine, even in some cases the animals were vaccinated with both types of vaccine.It should be noted that whichever the administered vaccine, it produces an observableresponse for up to thirty months.2,26,30,31
Although it has been mentioned that vaccination can provoke abortion, some authorsstate that in recently infected herds the animals present negative serology,for which it must be highlighted that in CAIT there were two animals that presentedvalues higher than 1:128 at the moment of abortion and showed suggestive respiratorysigns of IBR and were not vaccinated, what makes evident the circulation of thevirus in the studied area.2,8,29,32
The high titles of these and other found animals that presented abortion, mighthave occurred because they became infected at the place of origin, since it hasbeen found that the time elapse between the maternal and fetal infection is variableand can fluctuate from eight to several months.33
It is concluded thatIP and direct IF were not fast and dependable methods for the detection of bovine herpesvirus in fetal tissue. Both tests had a limited Sp for detecting the virus; itis worth noting that the IP as the IF resulted in less costly and difficult methodsthan VI for the detection of bovine herpes virus. Other variables that may alterthe test results are ; the time that passes since the sample is taken until thediagnostic test is done, the autolysis, the quality of the conjugate , time ofvaccination and the moment of abortion where the viral excretion is minimal.
There was no relation found between the results of IP and IF in regard to thoseof the SN; the SN test has been internationally accepted as the technique ofreference; therefore, it is necessary to define the endemic condition of areawhere it is used.
It is necessary to continuewith the studies to increase the dependability of the diagnostic tests, likethe imunoperoxidase in plate and avidin-biotin techniques, in order toreduce the false positives due to the effect of red corpuscles present in thetissues, it is also necessary to evaluate the behavior of the antibodies in agroup of infected animals and immunized during all the stages of the disease,recuperation stage and reactivation of the virus in different dairy zones, todetermine the relation of the results with the evolution of the disease and tospecify the best test for its diagnosis.
Acknowledgments
Special thanks for the technical support of the National Center ofDiagnostic Services in Animal Health (CENASA) and the Virology and PathologyLaboratories during the practical elaboration of this study; likewise, we acknowledgethe participation of the Agricultural and Industrial Complex of Tizayuca (CAIT),Tizayuca, Hidalgo: Pathology and Diagnosis of Animal Health, for the obtainmentof the samples.
Table 1In relation with IP characteristics as diagnostic test, it was found that Se was greater (96.7%) to the one found by Smith et al. and Delgado et al.,15,17 who determined a Se of 96% for avidin-biotin and 83.3% by direct technique, respectively. Nevertheless, the Sp found (20%) was less than the notified by Delgado et al.,15 which was nearer to 100%. The high quantity of positive false derived from the low specificity, was probably due to a focal unspecific coloration that the sample proportionates and gives the morphology of the blood vessels and the presence of red blood cells in this, as it has been mentioned by Smith12 and Theodoris et al.25 Another possible explanation is that the serum or the sample, or both, were contaminated with other type of proteins, such as the bacteriological origin and to the possible cross reaction with other herpes viruses.26 Another cause in the variation of the results could be due to a low concentration of serum antibodies that is used to reveal the presence of the antigen, as Kramps mentions.3 Furthermore, the coloration can not be visible if the concentration in the tissue is minimal, impeding that the conjugate detects its presence, as mentioned by Delgado and Kelling.15 26
The IP performed in plate to confirm the VI favors the possibility to find theantigen, since, for the elaboration of this technique, it is necessary to performa maceration of all the organs (pool), while the direct technique of the frozencut does not guarantee, in the evaluated tissue portion, that the antigen isin seropositive animals. Posposil et al 27 have demonstrated the presenceof the virus in liver, kidney, lung and other aborted fetus organs of seronegativeanimals and in some experimentally infected seropositives. In sever cases ofautolysis , the samples could not be evaluated by IP neither for IF, becausethe tissue detached itself at the moment of washing during the techniqueprocess.
In the cuts of frozentissue, the anatomy of the evaluated organ was not always seen due to the autolysis,this condition did not interfere with the interpretation of the IP test, becausethe obtained coloration in the positive cases was evident; therefore, all thetissues could be evaluated, not so with IF in which, in autolysis conditions,unspecific redish coloration was observed, difficulting the interpretation ofthe results, which coincides with Theodoris et al and Reed etal.25,28. It is to be mention that the samples that did not presented severeautolysis, the encounter fluorescence was focal and dim uniformly distributedin the nucleus and cytoplasm, specifically in kidney, which coincides with thefinding by Reed et al.28
The Se and Sp obtained by IF (24.1%, 70%) were lower to ones found by Delgado etal and Smith et al 15,17 whom obtained Se of 67% and 83.3%, respectively,not so with Sp, since Delgado et al 15 found 100% under laboratory conditions.The lower Se could be due to the autolysis of the samples, as well as the samereasons described for IP and the treatment given to the sample, which can affectthe antigenic site and the quality of the antibodies in the serum, in order toreveal the presence of the antigen.26
The correlation between the VI and IF found by Reed et al 28 was of 50%. Onthe other hand, Bratanich et al 14 mention a concordanceof 83%, which is higher than the one found in the present study of only 42.9%.
In this study the organsof election for the samples send to the laboratory were: liver, kidneyand lung, which coincide with the indicated by Smith et al 17
The variation in the values of Sn, which go from 1:2 to higher than 1:128, incows at the moment of abortion, coincide with the indicated by S. Van Drunen etal. 29 A problem with the serological evaluation was trying to differentiatethe positive serums, since antibodies induced by natural infection or vaccination couldbe found, most of all if it is considered that the titles can be similar to vaccinationas to natural infection.30 It is important to point out that in CAIT, atthe beginning of the sampling, some animals were inoculated with thermosensibleattenuated vaccine and during the study every cow received inactivated or modifiedvaccine, even in some cases the animals were vaccinated with both types of vaccine.It should be noted that whichever the administered vaccine, it produces an observableresponse for up to thirty months.2,26,30,31
Although it has been mentioned that vaccination can provoke abortion, some authorsstate that in recently infected herds the animals present negative serology,for which it must be highlighted that in CAIT there were two animals that presentedvalues higher than 1:128 at the moment of abortion and showed suggestive respiratorysigns of IBR and were not vaccinated, what makes evident the circulation of thevirus in the studied area.2,8,29,32
The high titles of these and other found animals that presented abortion, mighthave occurred because they became infected at the place of origin, since it hasbeen found that the time elapse between the maternal and fetal infection is variableand can fluctuate from eight to several months.33
It is concluded thatIP and direct IF were not fast and dependable methods for the detection of bovine herpesvirus in fetal tissue. Both tests had a limited Sp for detecting the virus; itis worth noting that the IP as the IF resulted in less costly and difficult methodsthan VI for the detection of bovine herpes virus. Other variables that may alterthe test results are ; the time that passes since the sample is taken until thediagnostic test is done, the autolysis, the quality of the conjugate , time ofvaccination and the moment of abortion where the viral excretion is minimal.
There was no relation found between the results of IP and IF in regard to thoseof the SN; the SN test has been internationally accepted as the technique ofreference; therefore, it is necessary to define the endemic condition of areawhere it is used.
It is necessary to continuewith the studies to increase the dependability of the diagnostic tests, likethe imunoperoxidase in plate and avidin-biotin techniques, in order toreduce the false positives due to the effect of red corpuscles present in thetissues, it is also necessary to evaluate the behavior of the antibodies in agroup of infected animals and immunized during all the stages of the disease,recuperation stage and reactivation of the virus in different dairy zones, todetermine the relation of the results with the evolution of the disease and tospecify the best test for its diagnosis.
Acknowledgments
Special thanks for the technical support of the National Center ofDiagnostic Services in Animal Health (CENASA) and the Virology and PathologyLaboratories during the practical elaboration of this study; likewise, we acknowledgethe participation of the Agricultural and Industrial Complex of Tizayuca (CAIT),Tizayuca, Hidalgo: Pathology and Diagnosis of Animal Health, for the obtainmentof the samples.
EVALUATION OF 49 SAMPLES OF ABORTED FETUSES BY THE IMMUNOFLUORESCENCE, IMMUNOPEROXIDASEAND VIRAL ISOLATION TESTS FOR IBR, CAIT; MEXICO, 1997

Table 2
IBR ANTIGEN DETECTION BY IMMUNOFLUORESCENCE AND IMMUNOPEROXIDASE, IN ORGANS OF ABORTED FETUSES. CAIT; MEXICO, 1997
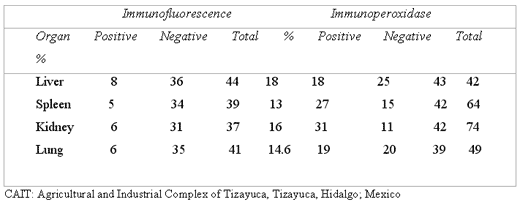
Table 3
SENSIBILITY, SPECIFICITY AND PREDICTIVE VALUE OF THE IMMUNOFLUORESCENCE AND IMMUNOPEROXIDASE TESTS AND THEIR CONCORDANCE WITH THE VIRAL ISOLATION TEST, CAIT; MEXICO, 1997
CAIT = Agricultural and Industrial Complex of Tizayuca, Tizayuca, Hidalgo; Mexico.
Se = Sensibility
Sp = Specificity
PV(+) = Positive predictive value
PV(-) = Negative predictive value
GCI = Gross concordance index
K I = Kappa index
p = Interval of confidence
Se = Sensibility
Sp = Specificity
PV(+) = Positive predictive value
PV(-) = Negative predictive value
GCI = Gross concordance index
K I = Kappa index
p = Interval of confidence
Table 4
SENSIBILITY, SPECIFICITY AND PREDICTIVE VALUE TESTS OF IMMUNOFLUORESCENCE AND IMMUNOPEROXIDASE AND THEIR CONCORDANCE WITH VIRAL ISOLATION PER ORGAN, CAIT, TIZAYUCA – HIDALGO; MEXICO, 1997
CAIT = Agricultural and Industrial Complex of Tizayuca, Tizayuca, Hidalgo; Mexico.
Se = Sensibility
Sp = Specificity
PV(+) = Positive predictive value
PV(-) = Negative predictive value
K I = Kappa index
p = Interval of confidence
Se = Sensibility
Sp = Specificity
PV(+) = Positive predictive value
PV(-) = Negative predictive value
K I = Kappa index
p = Interval of confidence
Table 5
RESULTS OF SENSIBILITY, SPECIFICITY AND POSITIVE AND NEGATIVE PREDICTIVE VALUES OF SERONEUTRALIZATION AND THEIR CONCORDANCE WITH VIRAL ISOLATION, CAIT; MEXICO, 1997
CAIT = Agricultural and Industrial Complex of Tizayuca, Tizayuca, Hidalgo; Mexico.
Se = Sensibility
Sp = Specificity
PV(+) = Positive predictive value
PV(-) = Negative predictive value
K I = Kappa index
p = Interval of confidence
Se = Sensibility
Sp = Specificity
PV(+) = Positive predictive value
PV(-) = Negative predictive value
K I = Kappa index
p = Interval of confidence
Related topics:
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.



