Vaccine Preperative Trial for Leptospirosis and their Pathological, Immunological Study by Serum Electrophoresis
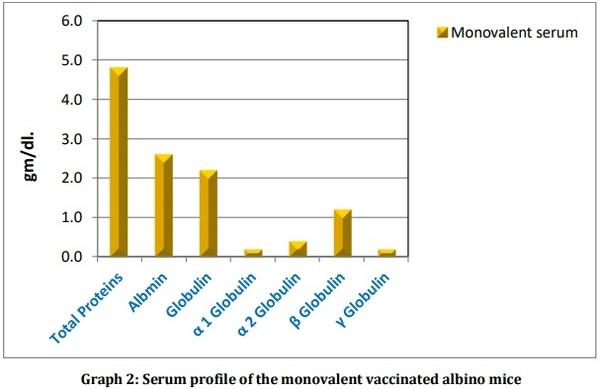
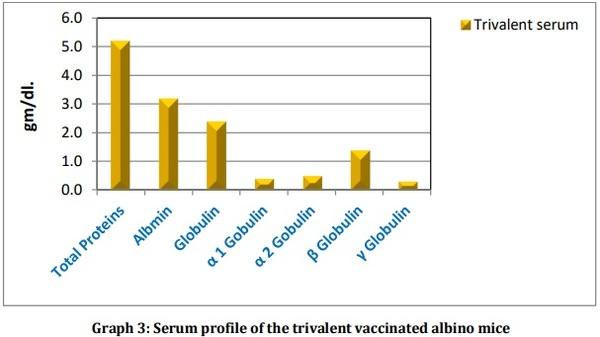
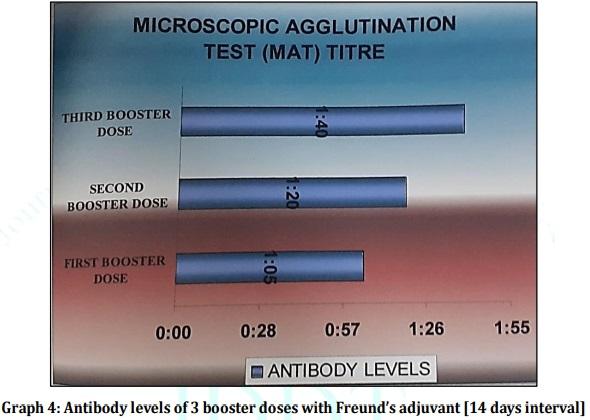
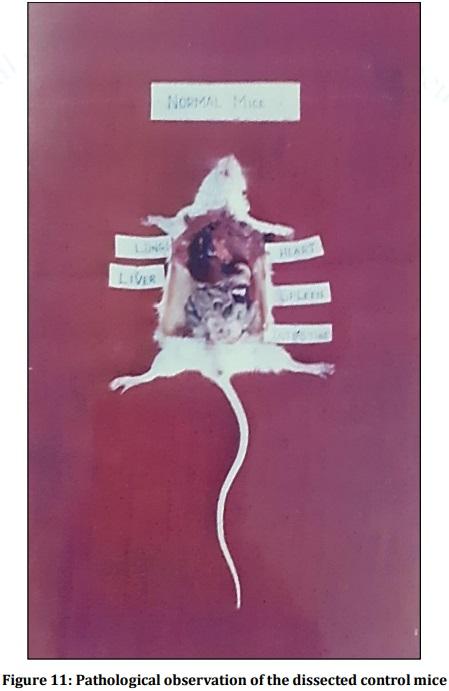
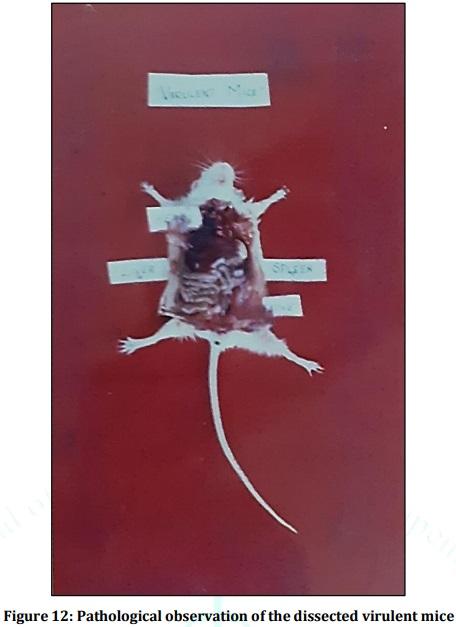
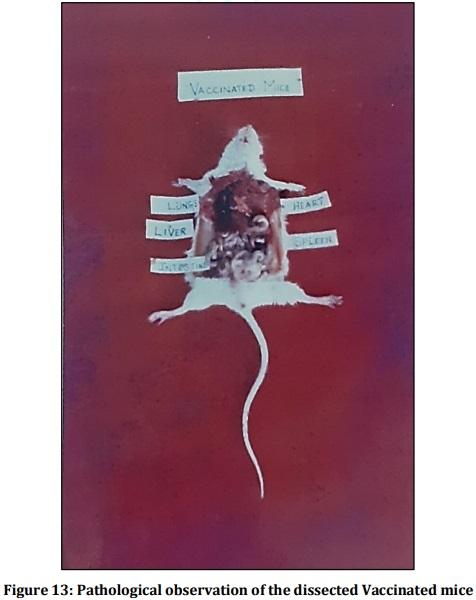
Leptospirosis is a fatal infectious disease caused by different serovars of Leptospira spirochetes affecting humans and animals. In the present study, the trials of the whole-cell killed formalin treated monovalent vaccine using Leptospira icterohaemorrhagiae and trivalent vaccine using Leptospira icterohaemorrhagiae, Leptospira louisiana, and Leptospira hebdomadis were studied. The serum electrophoresis studies were done after administration of the vaccine into the experimental albino mice along with the booster dose of the vaccinated serum by densitometric readings. Similarly, the pathological observations were made by dissecting the virulent mice, vaccinated mice, and comparing them with the control mice. The MAT titre was also studied after the booster dose administration of the vaccinated serum. The monovalent and trivalent whole-cell killed formalin treated vaccines shows significant raise in the total proteins, albumin, globulin, α 1 globulin, α 2 globulin, β globulin and γ globulins of the serum as well as increase in significant levels in the antibody levels after the administration of the booster dose at an interval of 14 days.
Keywords: Leptospira, Whole-cell killed formalin treated vaccine, Immunological study, Pathological study, Serum electrophoresis.
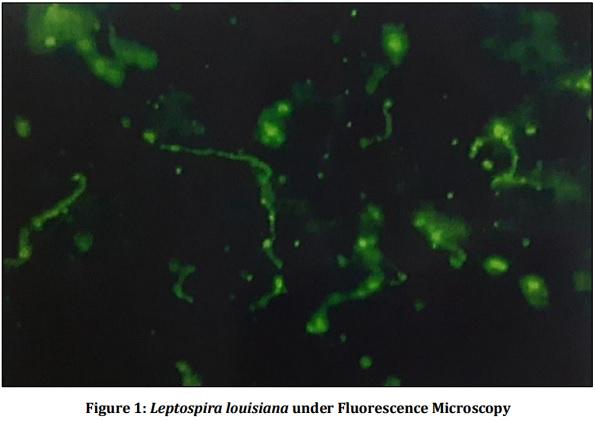
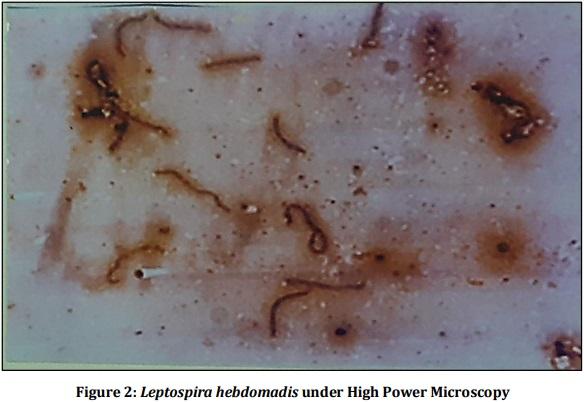
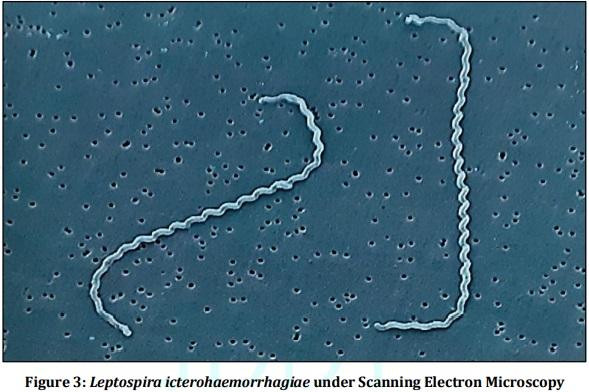
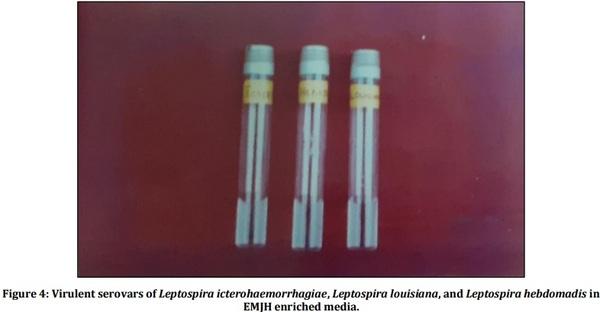

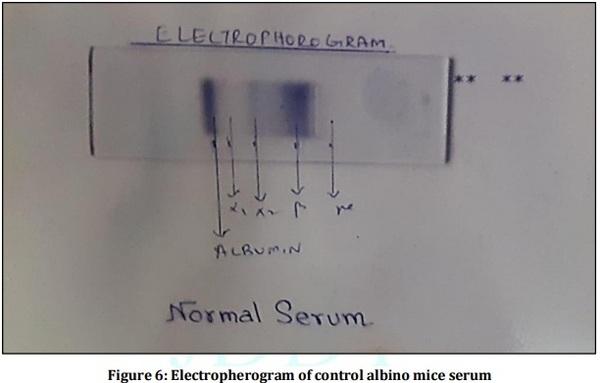
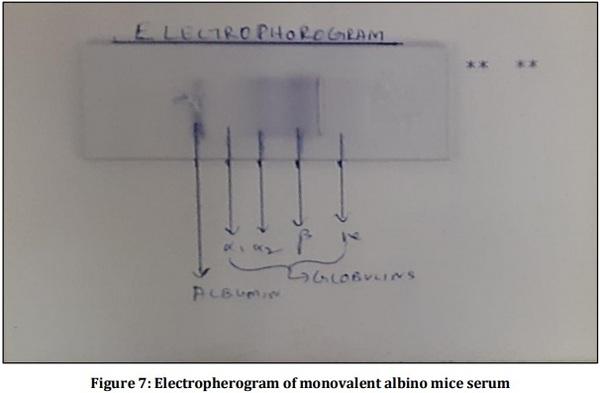
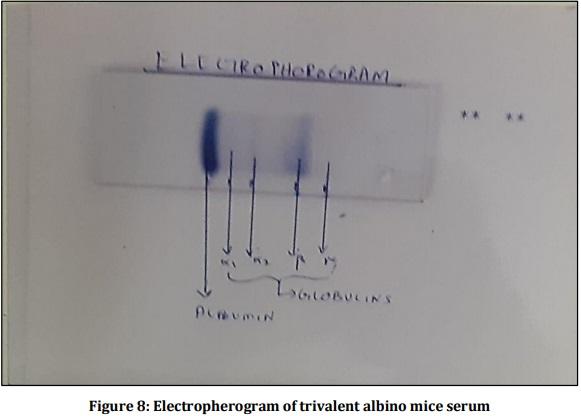
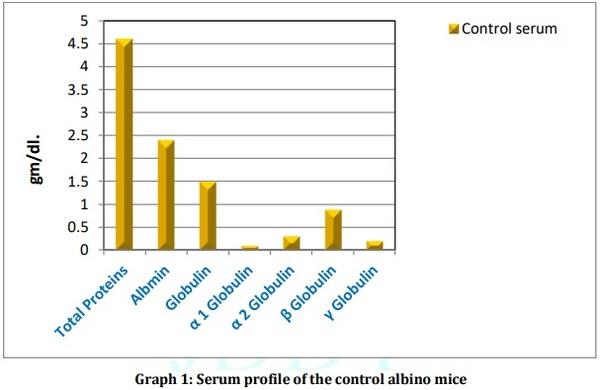

[01] Alison BL, Michael MD, “Leptospirosis: A clinical review of evidence based diagnosis, treatment and prevention” World J
Clin Infect Dis, 2016; 6(4):61-66.
[02] Clinton KM, Michael WE, Duaner H, “Susceptibility of
Leptospira serovars to antimalarial agents” Am. J. Trop. Med.
Hyg, 2004; 71(5):685–686.
[03] Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, MartinezSilveira MS, Stein C, Abela-Ridder B, Ko AI, “Global Morbidity and Mortality of Leptospirosis: A Systematic Review” PLoS Negl
Trop Dis, 2015; 9:e0003898.
[04] Ayyar VK, “A note on the outbreak of leptopspiral jaundice among madras bounds” Indian J Vet Sci, 1932; 2:169.
[05] Adinarayanan N, Jain NC, Chandiramani HK, Haleja SK, “Studies on leptospirosis among bovines in India: A preliminary report on the occurrence in cattle based on serological evidence”
Indian Vet J, 1960; 37:251.
[06] Sane CR, Desphande BR, “Record of detection of Leptospira
Pomona infection as a cause of abortion in calves –letters to the editor. IVJ” Indian Vet J, 1965; 42:75.
[07] Rajasekhar M, Nanjiah RD, “Animal leptospirosis in Mysore state: A serological study” India Vet J, 1971; 48:1087.
[08] Srivastava SK, Singh SP, Srivastava NC, “Seroprevalance of leptospirosis in animals and man in India” Indian J Comp
Microbiol Immunol Infect Dis, 1983; 4:243.
[09] David AH, Paul NL, “Leptospirosis in Humans” Curr Top
Microbiol Immunol, 2015; 387:65–97.
[10] McBride AJ, Athanazio DA, Reis MG, Ko AI, “Leptospirosis” Curr
Opin Infect Dis, 2005; 18:376-386.
[11] Looke DF, “Weil's syndrome in a zoologist” Med J Aust, 1986;
144:600-601.
[12] Trueba G, Zapata S, Madrid K, Cullen P, Haake D, “Cell aggregation: a mechanism of pathogenic Leptospira to survive in fresh water” Int Microbiol, 2004; 7:35-40.
[13] Levett PN, “Leptospirosis” Clin Microbiol Rev, 2001; 14:296-
326.
[14] Kuriakose M, Eapen CK, Paul R, “Leptospirosis in Kolenchery,
Kerala, India: epidemiology, prevalent local serogroups and serovars and a new serovar” Eur J Epidemiol, 1997; 13:691-697.
[15] Waitkins SA, “Leptospirosis as an occupational disease” Br J Ind
Med, 1986; 43:721-725.
[16] WHO. Human leptospirosis: guidance for diagnosis, surveillance, and control. Switzerland: WHO, Geneva, 2003.
[17] Dolhnikoff M, Mauad T, Bethlem EP, Carvalho CR, “Leptopspiral pneumonias” Curr Opin Pulm Med, 2007; 13:230-235.
[18] Bethlem EP, Carvalho CR, “Pulmonary leptospirosis” Curr Opin
Pulm Med, 2000; 6:436-441.
[19] Souza AL, Sztajnbok J, Marques SR, Seguro AC, “Leptospirosis induced meningitis and acute renal failure in a 19-month-old male child” J Med Microbiol, 2006; 55:795-797.
[20] Daher E, Zanetta DM, Cavalcante MB, Abdulkader RC, “Risk factors for death and changing patterns in leptospirosis acute renal failure” Am J Trop Med Hyg, 1999; 61:630-634.
[21] Morsi HM, Shibley GP, Strother HL, “Renal leptospirosis: challenge exposure to vaccinated and non-vaccinated cattle to
Leptospira icterohaemorrhagiae and Leptospira canicola” Am J
Vet Res, 1973; 34:175-179.
[22] Schreiber P, Martin V, Najbar W, Sanquer A, Gueguen S, Lebreux
B, “Prevention of renal infection and urinary shedding in dogs by a Leptospira vaccination” Vet Microbiol, 2005; 108:113-118.
[23] Broughton ES, Scarnell J, “Prevention of renal carriage of leptospirosis in dogs by vaccination” Vet Rec, 1985; 117:307-
311.
[24] Adamus C, Buggin-Daubie M, Izembart A, Sonrier-Pierre C,
Guigand L, Masson MT, Andre-Fontaine G, Wyers M, “Chronic hepatitis associated with leptospiral infection in vaccinated beagles” J Comp Pathol, 1997; 117:311-328.
[25] Spichler A, Spichler E, Moock M, Vinetz JM, Leake JA, “Acute pancreatitis in fatal anicteric leptospirosis” Am J Trop Med Hyg,
2007; 76:886-887.
[26] Derham RL, Owens GG, Wooldridge MA, “Leptospirosis as a cause of erythema nodosum” Br Med J, 1976; 2:403-404.
[27] Katz AR, Ansdell VE, Effler PV, Middleton CR, Sasaki DM,
“Assessment of the clinical presentation and treatment of 353 cases of laboratory-confirmed leptospirosis in Hawaii, 1974–
1998” Clin Infect Dis, 2001; 33:1834-1841.
[28] Christova I, Tasseva E, Manev H, “Human leptospirosis in
Bulgaria, 1989–2001: epidemiological, clinical, and serological features” Scand J Infect Dis, 2003; 35:869-872.
[29] Koizumi N, Watanabe H, “Leptospirosis Vaccines: Past, Present and Future” J Postgrad Med, 2005; 51(3):210-214.
[30] Zhijun W, Li J, Alicja W, “Leptospirosis vaccines” Microbial Cell
Factories, 2007; 6:39.
[31] Babudieri B, “Vaccine against Leptospirosis” Fifth international meeting of biological standardization. Science press of Israel.
Jerusalem, the Weizmann; 1959 p 313-350.
[32] Chen TZ, “Development and present state of leptospiral vaccine and technology of vaccine production in china” Jap J Bacteriol,
1985; 40:755-762.
[33] Sanchez RM, Sierra AP, Suarez MB, Alvarez AM, Hernandez JM,
Gonzalez MD, “Evaluation of the effectiveness of new vaccine against human leptospirosis in groups at risk” Rev Panam Salud
Publica, 2000; 8:385-392.
[34] Martinez R, Perez A, Quinones M, Cruz R, Alvarez A, Armesto M,
“Efficacy and safety of a vaccine against human leptospirosis in
Cuba” Rev Panam Salud Publica, 2004; 45:249-255.
[35] Haake DA, Walker EM, Blanco DR, Bolin CA, Miller MN, Lovett
MA, “Changes in the surface of Leptospira interrogans serovar grippotyphosa during Invitro cultivation” Infect Immun, 1991;
59:1131-1140.
[36] Haake DA, Champion CI, Martinich C, Shang ES, Blanco DR,
Miller JN, “Molecular cloning and sequence analysis of the gene encoding OmpL 1, a trans memmbrane outer protein of pathogenic Leptospira spp” J Bacteriol, 1993; 175:4225-4234.
[37] Shang ES, Summers TA, Haake DA, “Molecular cloning and sequence analysis of the gene encoding LipL41, a surface exposed Lipoprotein of pathogenic Leptospira spp” Infect
Immun, 1996; 64:2322-2330.
[38] Haake DA, Martinich C, Summers TA, Shang ES, Pruetz JD,
McCoy AM, “Characterization of leptospiral outer membrane lipoprotein Lip L36: Down regulation associated with late-log phase growth and mammalian infection” Infect Immun, 1998;
66:1579-1587.
[39] Park SH, Ahn BY, Kim MJ, “Expression and immunologic characterization of recombinant heat shock protein 58 of
Leptospira species: a major target antigen of humoral immune response” DNA Cell Biol, 1999; 18:903-910.
[40] Haake DA, Mazel MK, McCoy AM, Milward F, Chao G, Matsunaga
J, “Leptospiral outer membrane proteins OmpL 1and Lip L41 exhibit synergistic immunoprotection” Infect Immun, 1999;
67:6572-6582.
[41] Haake DA, Chao G, Zuerner RL, Barnett JK, Barnett D, Mazel M,
“The leptospiral major outer membrane protein Lip L32 is a lipoprotein expressed during mammalian infection” Infect
Immun, 2000; 68:2276-2285.
[42] Guerrerio H, Croda J, Flannery B, Mazel M, Matsunaga J, Galvao
RM, “Leptospiral proteins recognized during the humoral immune response to Leptospirosis in humans” Infect Immun,
2001; 69:4958- 4968.
[43] Branger C, Sonrrier C, Chatrenet B, Klonjkowski B, RuvoenClouet N, Aubert A, “Identification of the haemolyis associated protein 1as a cross protective immunogen of Leptospira interrogans by adenovirus mediated vaccination” Infect Immun,
2001; 69:6831- 6838.
[44] Nally JE, Artiushin S, Timoney JF, “Molecular characterization of thermo induced immunogenic proteins Q1p42 and Hsp 15 of
Leptospira interrogans”. Infect Immun, 2001; 69: 7616- 7624.
[45] Matsunaga J, Young TA, Barnett JK, Barnett D, Bolin CA, Haake
DA, “Novel 45 Kda leptospiral protein that is processed to 31
Kda growth phase regulated peripheral membrane protein”
Infect Immun, 2002; 70:323-324.
[46] Cullen PA, Cordwell SJ, Bulach DM, Haake DA, Adler B, “Global analysis of outer membrane proteins from Leptospira interrogans serovar Lai” Infect Immun, 2002; 70:2311- 2318.
[47] Haake DA, Matsunaga J, “Characterization of the leptospiral outer membrane and description of the three novel leptospiral membrane proteins” Infect Immun, 2002; 70:4936- 4945.
[48] Palaniappan RU, Chang YF, Jusuf SS, Artiushin S, Timoney JF,
McDonough SP, “Cloning and molecular characterization of immunogenic Lig A protein of Leptospira interrogans” Infect
Immun, 2002; 70:5924-5930.
[49] Koizumi N, Watanabe H, “Identification of novel antigen of pathogenic Leptospira spp. That reacted with the convalescent
Mice sera” J Med Microbiol, 2003; 52:585-589.
[50] Koizumi N, Watanabe H, “Molecular cloning and characterization of a novel leptospiral lipoprotein with OmpA domain” FEMS Microbiol Lett, 2003; 226:215-219.
[51] Cullen PA, Haake DA, Bulach DM, Zuerner RL, Adler B, “Lip L21 is a novel surface exposed lipoprotein of pathogenic Leptospira species” Infect Immun, 2003; 71:2414-2421.
[52] Matsunaga J, Barocchi MA, Croda J, Young TA, Sanchez Y,
Siqueria I, “Pathogenic Leptospira species express surface exposed proteins belonging to the bacterial immunoglobulin superfamily” Mol Microbiol, 2003; 49:929-945.
[53] Artiushin S, Timoney JF, Nally J, Verma A, “Host inducible immunogenic sphingomyelinase-1ike protein, LK73.5, of
Leptospira interrogans” Infect Immun, 2004; 72: 742-749.
[54] Koizumi N, Watanabe H, “Leptospiral immunoglobulin like proteins elicits protective immunity” Vaccine, 2004; 22:1545-
1552.
[55] Cullen PA, Haake DA, Adler B, “Outer membrane proteins of pathogenic spirochetes” FEMS Microbiol Rev, 2004; 28:291-318.
[56] Hsieh WJ, Chang YF, Chen CS, Pan MJ, “Omp52 is a growth phase regulated outer membrane protein of Leptospira santarosai serovar Shermani” FEMS Microbiol Lett, 2005; 243: 339-345.
[57] Nally JE, Whitelegge JP, Aguilera R, Pereira MM, Blanco DR,
Lovett MA, “Purification and proteomic analysis of outer membrane vesicles from clinical isolate of Leptospira interrogans serovar Copenhageni” Proteomics, 2005; 5:144-
152.
[58] Elliott SH, “Discussion and clinical diagnosis of Leptospirosis” J
Am Med Tech 1980: 42:37-44.
[59] Faine S, “Guidelines for the control of leptospirosis’ W. H. O.
Offset publication no. 67. World Health Organization, Geneva.
1982.
[60] Weyant RS, Bragg SL, Kaufmann AF, Leptospira and Leptonema, p. 739-745. In Murray, P.R., E.J. Baron, M.A. Pfaller, F.C. Tenover and R.H. Yolken (Ed.). Manual of clinical microbiology, 7th ed.
ASM Press, Washington, D.C. 1999.
[61] Ellinghausen HC, McCullough WG, “Nutrition of Leptospira pomona and growth of 13 other serotypes: fractionation of oleic albumin complex (OAC) and a medium of bovine albumin and polysorbate 80” Am J Vet Research, 1965; 26:45-51.
[62] Johnson R, Harris VG, “Differentiation of pathogenic of leptospires” J Bacteriol, 1967; 94:27-31.
[63] Rule PL, Alexander AD, “Gellan gum as a substitute for agar in leptospiral media’ J Clin Microbiol, 1986; 23:500-504.
[64] Isenberg HD, Clinical microbiology procedures handbook.
American Society for Microbiology, Washington, D.C. (Ed.).
1992.
[65] Koneman EW, Allen SD, Dowell VR, Janda WM, Sommers HM,
Winn WC, Color atlas and textbook of diagnostic microbiology,
3rd ed. J.B. Lippincott Company, Philadelphia, PA. 1988.
[66] Summaiya M, Tanvi HP, “Polymerase Chain Reaction: An
Important Tool for Early Diagnosis of Leptospirosis Cases” J Clin
Diagn Res, 2016; 10(12): DC08–DC11.
[67] Torten M, Shenberg E, Gerichter CB, Neuman P, Klingberg MA,
“A new leptospiral vaccine for use in man. II. Clinical and serologic evaluation of a field trial with volunteers” J Infect Dis,
1973; 128:647- 651.
[68] Nomura S, Kishie B, Yoshikawa H, Noro M, Sakurai N, Akatsuka
Y, “Case reports on Weil’s disease in Kumano district, Mie prefecture and vaccination (Japanese in original)” Media-circle,
1971; 40:16-25.
[69] EUROPEAN PHARMACOPOEIA (2002a). Monograph
01/2002:0447: Leptospira vaccine for veterinary use.
EuropeanDirectorate for the Quality of Medicines and
HealthCare (EDQM), Council of Europe, Strasbourg, France, p.
2270.
[70] Kitaoka M, Inoue S, “Standard procedures of Weil’s disease vaccine and Weil’s disease and akiyami combined vaccine (Japanese in original)” Jpn Med J, 1952; 1478:2845- 2847.
[71] Lindblad EB, Freund's Adjuvants. In: O’Hagan D.T. (eds) Vaccine
Adjuvants. Methods in Molecular Medicine™, vol 42. Springer,
Totowa, NJ. (2000).
[72] Christine B, Adriana G, George CK, “Drawing Blood from Rats through the Saphenous Vein and by Cardiac Puncture” J Vis Exp,
2007; (7):266.
[73] Gorg AW, Postel S, Gunther, Whitaker, “Improved horizontal two-dimensional electrophoresis with hybrid isoelectric focusing in immobilized pH gradients in the first dimension and laying-on transfer to the second dimension”
Electrophoresis, 1985; 6:599–60.
[74] Brandao AP, Camargo ED, da Silva ED, Silva MV, Abrao RV,
“Macroscopic Agglutination Test for Rapid Diagnosis of Human
Leptospirosis” J Clin Microbiol, 1998; 36(11):3138-3142.
[75] Gomes-Solecki M, Santecchia I, Werts C, “Animal Models of
Leptospirosis: Of Mice and Hamsters” Front Immunol, 2017;
8:58.
[76] White FH, Simpson CF, “The effect of formalin and other inactivators on the ultrastructure of leptospires” J Infect Dis,
1965; 115: 123-130.
[77] Suizer CR, Jones WL, Leptospirosis: methods in laboratory diagnosis. U.S. Department of Health, Education, and Welfare publication no. (CDC) 76-8275, p. 12-15. Center for Disease
Control, Atlanta. 1980.
[78] Chen TZ, “Development and the present status of leptospiral vaccine and technology of vaccine production in china” Jap J
Bacteriol, 1985; 40:755-762.
[79] Philip NA, Tennent RB, “Leptospirosis: a report on one practice on the use of a leptospiral vaccine for a period of 3 years” N Z med J, 1966; 65:13-19.
[80] In: Faine S, Adler B, Bolin C, Perolat P. Leptospira and
Leptospirosis, 2nd edn. MediSci: Melbourne; 1999.
[81] Naiman BM, Alt D, Bolin CA, Zuerner R, Baldwin CL, “Protective killed Leptospira borgpetersenii vaccine induces potent Th1 immunity comprising responses by CD4 and gammadelta T lymphocytes” Infect Immun, 2001; 69(12):7550-7558.
[82] Samina I, Brenner J, Moalem U, Berenstein M, Cohen A, Peleg B,
“Enhanced antibody response in cattle against Leptospira hardjo by intradermal vaccination” Vaccine, 1997; 15(12-13):1434-1436.







.jpg&w=3840&q=75)