The use of dietary antioxidants to extend colour shelf life in farmed juvenile Southern Bluefin Tuna (Thunnus maccoyii)
This research on the quality of farmed Southern Bluefin Tuna (SBT) (Thunnus maccoyii) has been focused on improving the colour shelf life of the sashimi product. Previous outcomes of our research group and supporting data from associated project groups have led to the current interest in evaluating the use of a supplementary feed for tuna farming that can be used to extend shelf life.
As with terrestrial red meats, shelf life is an important product quality feature of farmed SBT. Also in common with beef, the bright red colour of SBT is due to the myoglobin content of the meat. During storage, the myoglobin is oxidised to met-myoglobin and gradually changes from red to brown.
In addition and unlike beef, the high levels of highly unsaturated fatty acids found in SBT meat provide a strong oxidative pressure that can increase the rate of post-mortem browning.
Increasing the tissue level of α-tocopherol (vitamin E), ascorbic acid (vitamin C) or selenium, alone or in combinations, has been shown to successfully extend the shelf life of beef (Sanders et al., 1997), pork (Houben, 1998) and fish (Baker, 1997; Bell and Cowey, 1985; Hemre et al., 1997). These nutrients have natural antioxidant properties, which aid in the inhibition of the oxidation of fatty acids, development of off flavours and browning.
By slowing the oxidation process in SBT meat it is possible to extend the window of sale opportunity at the market and reduce the losses that may currently be experienced through the practice of trimming browned meat during cold storage of portioned carcasses. These improvements obviously have the capacity to improve the reputation of this valuable product in what is becoming an increasingly competitive export market.
From the 2001 farming season up to and including the 2004 season, our research group ran experiments designed to investigate and evaluate the use of feeds (baitfish and pelleted) fortified with the natural antioxidants dl-α-tocopherol acetate, l-ascorbic acid phosphate and selenium (as sodium selenate in 2001 or organoselenium from Sel-Plex® selenium yeast (Alltech Inc.) in the following years).
The aims of the research were first, to determine if feeding diets fortified with these antioxidants would result in a muscle dose response and second, whether the colour shelf life of the product could be extended.
Methods Individual pontoons of fish were fed either standard commercial diets or diets fortified with antioxidants. Details of the treatments for each farming season (2001-2004) are elaborated below with the respective muscle tissue dose response results reported.
FISH HUSBANDRY
SBT used in this research were purse-seined by commercial tuna operators in the Great Australian Bight. These fish were subsequently held in pontoons near Boston Bay, Port Lincoln, South Australia.
To determine the vitamin C, vitamin E, and selenium concentrations in the muscle of the SBT at the beginning of the experiment, tuna muscle samples were collected from 10 harvested fish. The concentration of antioxidants in these samples was not included in the statistical comparison as they were obtained in order to give an indication of the start point for the experiment.
FISH SAMPLING
Immediately after net harvest, fish were quickly killed following standard industry practice (Thomas et al., 2003). Following slaughter a muscle sample was excised from the fish using a 17 mm stainless steel coring tool inserted into the bleed wound of each fish. A portion of the resulting muscle was immediately frozen in liquid nitrogen with the remainder stored on ice for post-mortem colour assessment.
ANALYTICAL PROCEDURES
Muscle collected from each SBT was analysed for concentrations of vitamin C, vitamin E and selenium. The α-tocopheryl and dl-α-tocopheryl acetate concentrations were determined by HPLC based on the method of Huo (1999) modified for SBT muscle by D’Antignana et al. (2004).
Vitamin C was determined by a technique based on the HPLC fluorescence detection method of Brown and Miller (1992), which was adapted to SBT muscle in the laboratory at the Lincoln Marine Science Centre with the cooperation of Malcolm Brown of CSIRO Hobart, Tasmania.
Muscle samples analysed for selenium were sent stored in dry ice to Regional Laboratory Services, Benalla, Victoria, Australia.
Selenium concentration was determined using a method based on the fluorimetric technique of Watkinson (1966) modified as per Paynter et al. (1993).
Results and discussion
2001
In 2001 a preliminary trial using fortified pelleted diets (Table 1) demonstrated that SBT responded to added dietary antioxidants in that there was a dose response apparent in the muscle (Table 2) and that there was an increase in shelf life of the product.
In this trial sodium selenate was added to the pelleted diet, however there was no obvious uptake of this form of selenium measured in the fish muscle. In addition, although the change was small there was some evidence of a decline in fish muscle selenium concentration over the time of the trial, despite the addition of sodium selenate.
Table 1. Concentration of antioxidants added to SBT pelleted diets.
1Rovimix E–50
2Stay C 35
Table 2. Tissue level of antioxidants in SBT following 10 weeks of feeding.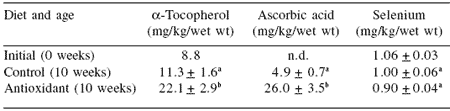
n.d. - Not determined.
abValues (n=10) that share the same superscript do not differ (P>0.05).
Initial values are not included in the comparison.
2002
In the following year a comparison was made between a ‘standard’ pellet diet, one fortified with vitamins E and C and selenium (Sel-Plex®, Alltech Inc.) and one fortified with vitamin E alone (Table 3).
Table 3. Concentration of antioxidants added to SBT pelleted diets.
1Rovimix E–50
2Stay C 35
As in 2001, there was a measurable increase in vitamin levels found in the muscle of fish fed the fortified diets, which could be directly attributed to the concentrations of vitamins added to the diets (Table 4). Unlike the result of 2001, muscle selenium concentration appeared to be maintained in fish fed organic selenium-fortified diets.
As in 2001, the shelf life of the fish flesh was extended in fish fed fortified diets and in this regard the diet fortified with vitamins E and C and selenium inhibited post-mortem change better than was achieved with the diet fortified with vitamin E alone.
Table 4. Tissue level of antioxidants in SBT after 10 weeks of feeding.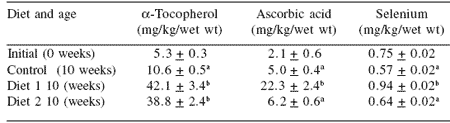
abValues (n=10) that share the same superscript do not differ (P>0.05).
Initial values are not included in the comparison.
2003
In 2003 the direction of the research changed slightly and a vitamin fortified baitfish diet was investigated. In this trial a standard baitfish diet was compared with baitfish fortified with vitamins E and C (Table 5). A proportion of the daily baitfish ration was coated with a vitamin plus gluten premix just prior to feeding. There was no added selenium used in this trial.
Table 5.Concentration of antioxidants added to SBT baitfish diets.
1Rovimix E–50
2Stay C 35
The results of this trial demonstrated that vitamin C could be delivered to the fish by using a gluten vitamin coating system. However the muscle dose response of vitamin E indicated that the delivery or uptake of this vitamin was poor using this method of diet fortification (Table 6).
Muscle selenium concentration was maintained in fish during this trial, however, it is important to note that there is evidence to suggest that muscle selenium declines in fish during capture and relocation to pens that occurs prior to ‘initial’ samples being taken for analysis (D’Antignana et al., 2004).
Table 6. Tissue level of antioxidants in SBT after 10 weeks of feeding.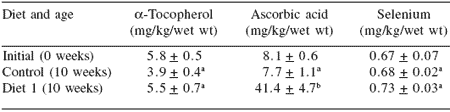
Values (n=10) that share the same superscript letter do not differ (P>0.05)
Initial values are not included in the comparison
2004
In 2004 a trial was conducted that compared four diets, two using baitfish and two using pellet diets. The standard baitfish and the standard pellet diets represented those currently being fed to farmed SBT as standard commercial practice.
The fortified diets were the same as the standard diets except for the addition of the antioxidants (Table 7). This design enabled us to measure the uptake of the antioxidants over approximately 18 weeks, compare the muscle dose response of the respective diets and concurrently the shelf life of the muscle following the completion of the trial.
Table 7.Concentration of antioxidants added to SBT baitfish and pelleted diets.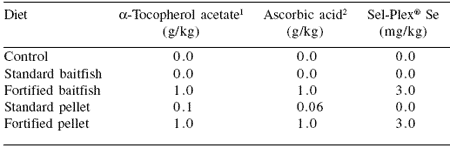
1Rovimix E–50
2Stay C 35
The muscle concentration of vitamin E prior to the trial was similar to that seen in ‘Initial’ samples from trials held in previous years. Fortified baitfish and fortified pellet diets resulted in the largest muscle dose response, with the fortified pellet diet performing much better than the fortified baitfish diet. The dose response of the fortified baitfish diet levelled off after approximately 8 weeks of feeding and this level was sustained for approximately 4 weeks after the cessation of treatment (Figure 1).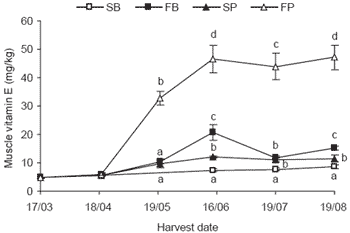
Figure 1.SBT muscle vitamin E concentration prior to treatment and at consecutive harvests over an 18 week trial (SB = standard baitfish, FB = fortified baitfish, SP = standard pellet, FP = fortified pellet). Treatment started 17/03/04 and ceased 19/07/04 prior to the last sampling 19/08/04. Values (n=10) at each date with the same letter do not differ (P>0.05).
The muscle concentration of vitamin C prior to the trial was similar to that seen in ‘Initial’ samples from trials held in previous years. Fortified baitfish and fortified pellet diets resulted in a similar muscle dose response that was much larger than the standard baitfish or standard pellet diets.
The dose response of the fortified baitfish and fortified pellet fed fish levelled off after approximately 4 weeks of feeding. Approximately 4 weeks after the cessation of treatment (19/07) there was a large drop (~50%) in the muscle level of vitamin C in both fortified diets (Figure 2).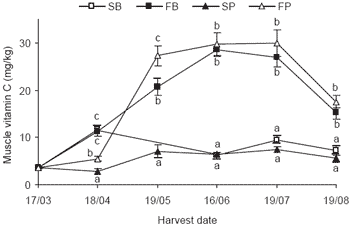
Figure 2.SBT muscle vitamin C concentration prior to treatment and at consecutive harvests over an 18 week trial (SB = standard baitfish, FB = fortified baitfish, SP = standard pellet, FP = fortified pellet). Treatment started 17/03/04 and ceased 19/07/04 prior to the last sampling 19/08/04. Values (n=10) at each date with the same letter do not differ (P>0.05).
The muscle concentration of selenium prior to the trial was similar to that seen in ‘Initial’ samples from trials held in previous years. The fortified pellet diet resulted in a measurable increase in the level of muscle selenium in SBT (Figure 3).
The muscle selenium level appeared to be maintained in the fish fed the standard baitfish or fortified baitfish diets but appeared not to be maintained in those fed the standard pellet diet. The dose response of the fortified baitfish appeared to increase up until the cessation of treatment and then decline slightly during the four weeks prior to the last harvest.
The shelf life of the flesh of the fish fed the fortified pellet diet was extended more than that of any of the other treatments with inhibition of post-mortem oxidation still visibly evident after 10 days of refrigerated storage (results not shown). The flesh from fish fed the fortified baitfish was the next best, and after 10 days storage there was a clear difference between the visual appearance of the flesh from the fish fed the fortified diets compared to those fed the standard diets.
Looking specifically at selenium; available data (Padula, unpublished) suggest that to date, muscle selenium levels in tuna fed fortified diets are within the normal range of the same species of wild caught tuna (Table 8).
Our results indicate that farmed tuna typically have half the muscle selenium content of wild-caught tuna. This is supported by the results of D’Antignana (2004). This information, in combination with recent research demonstrating the benefits of selenium and in particular organic selenium supplementation (Power, 2005; Surai, 2002) support the use of feeds fortified with organoselenium as Sel-Plex® for SBT and perhaps other aquaculture species.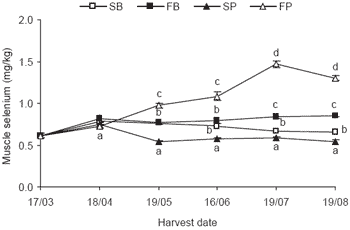
Figure 3. SBT muscle selenium concentration prior to treatment and at consecutive harvests over an 18 week trial (SB = standard baitfish, FB = fortified baitfish, SP = standard pellet, FP = fortified pellet). Treatment started 17/03/04 and ceased 19/07/04 prior to the last sampling 19/08/04. Values (n=10) at each date that share the same letter do not differ (P>0.05).
Table 8. Wild fish muscle selenium values.
2002 are tail meat values and 2004 are composite (Padula, unpublished).
| Conclusions Our research with SBT has examined the use of increased levels of dietary antioxidants to slow the browning process of tuna meat using fortified pellets and coated baitfish as a nutrient delivery system. We have established that feeding SBT pellets fortified with boosted levels of vitamins E and C and selenium will raise the level of these natural antioxidants in the fish muscle. Higher levels of these antioxidants in the muscle consistently result in an extension of the colour shelf life of sashimi grade tuna meat. We have also demonstrated that feeding SBT pellets fortified with a higher level of vitamin E alone is not as effective in extending the shelf life of SBT meat as a combination of higher levels of vitamins E and C plus selenium. In addition, we have shown that pellet diets fortified with antioxidants are by far the most effective delivery system for these nutrients and therefore the most effective way to improve the shelf life of farmed SBT flesh. |
Acknowledgments
This work formed part of a project of Aquafin CRC, and received funds from the Australian Government’s CRCs Program, the Fisheries R&D Corporation and other CRC Participants.
References
Baker, R.T.M. 1997. The effects of dietary α-tocopherol and oxidised lipid on post-thaw drip from catfish muscle. Anim. Feed Sci. Tech. 65:35-43.
Bell, J.G. and C.B. Cowey. 1985. Roles of vitamin E and selenium in the prevention of pathologies related to fatty acid oxidation in salmonids. In: Nutrition and Feeding in Fish (C.B. Cowey, A.M. Mackie and J.G. Bell, eds). Academic Press, London, pp. 333-347.
Brown, M.R. and K.A. Miller. 1992. The ascorbic acid content of eleven species of microalgae used in mariculture. J. Appl. Phycol. 4:205-215.
D’Antignana, T.D., P.M. Thomas and J. Carragher. 2004. The effect of different dietary combinations of vitamin E, vitamin C, and selenium on the distribution of these antioxidants in the carcasses of southern bluefin tuna (Thunnus maccoyii). In: Aquaculture Europe 2004, Biotechnologies for Quality. Barcelona, Spain, Oct 20-23, pp. 263-265.
Hemre, G., J.E. Juell, K. Hamre, L. Oyvind, B. Strand, P. Arnesen and J.C. Holm. 1997. Cage feeding of Atlantic mackerel (Scomber scombus): effect of lipid content, fatty acid composition, oxidation status and vitamin E concentration. Aquatic Living Resource 10:365- 370.
Houben, J.H., G. Eikelenboom and H.H. Bolink. 1998. Effect of the dietary supplementation with vitamin E on colour stability and lipid oxidation in packaged, minced pork. Meat Sci. 48:265-273.
Huo, J.Z., H.J. Nelis, P. Lavens, P. Sorgeloos, P. Andre and D. Leenheer. 1999. Simultaneous determination of α-trocopheryl acetate and tocopherols in aquatic organisms and fish feed. J. Chrom. 724:249-255.
Paynter, D.I., C.G. Halpin and I.W. Caple. 1993. Selenium nutrition. Assessment using glutathione peroxidase. Austr. Stand. Diagn. Techn. Anim. Dis. 45:1-7.
Power, R.F. 2005. A toxicological comparison of selenium sources: does enhanced bioavailability imply increased safety concerns? In: Nutritional Biotechnology in the Feed and Food Industries, Proceedings of the 21st Symposium (T.P. Lyons and K.A. Jacques, eds). Nottingham University Press, UK.
Sanders, S.K., J.B. Morgan, D.M. Wulf, J.D. Tatum, S.N. Williams and G.C. Smith. 1997. Vitamin E supplementation of cattle and shelf-life of beef for the Japanese Market. J. Anim. Sci. 75:2634-2640.
Surai, P.F. 2002. Natural antioxidants in avian nutrition and reproduction. Nottingham University Press, UK.
Thomas, P.M., C.G. Carter, J.F. Carragher and B.D. Glencross. 2003. Preliminary information on temporal changes in the blood chemistry of farmed southern bluefin tuna, Thunnus maccoyii (Castelnau), after feeding and repeated sampling disturbance. Aquacult. Res. 34:265-267.
Watkinson, J.H. 1966. Fluorometric determination of selenium in biological material with 2,3-diaminonaphthalene. Anal. Chem. 38:92-97.
Authors:P.M. THOMAS1 and J. BUCHANAN2
1 School of Biological Sciences, Flinders University and Aquafin Cooperative Research Centre, Port Lincoln, SA, Australia
2 SARDI and Aquafin Cooperative Research Centre, Port Lincoln, SA, Australia


.jpg&w=3840&q=75)