Nutritional needs for correct pigmentation in European red porgy (Pagrus pagrus)
The slow growth of the European sea bream and sea bass markets, together with progressive decreases in price and profit margins is leading to diversification of marine aquacultured species in the Mediterranean region. Red porgy (Pagrus pagrus), a close species to the widely cultured red sea bream (Pagrus major), is a fish highly appreciated for its nice appearance, firm flesh and delicate flavour, and brings prices over 20 €/kg in some European markets. Its range of distribution extends to both Atlantic coasts from the south of Britain to Angola including the Mediterranean, Adriatic Sea and Mexico.
Its natural diet in the wild includes decapod crustaceans, molluscs, small worms and fish. Wild catches of this species are very limited and insufficient to meet market demand. Therefore interest in developing culture techniques for this species has grown over the last 10 years (Hernández-Cruz et al., 1990, 1999; Kentouri et al., 1994), which has enabled its commercial production by fish farmers. However, under these culture conditions the natural colouring of red porgy is lost, and hence its market value accordingly reduced. Thus, whereas wild specimens exhibit a red-pink-silver colour, under captivity red porgy skin turns dark grey (Kentouri et al., 1995; Stephanou et al., 1995). Maintenance of the natural skin pigmentation is of great importance from a commercial point of view, as it has a direct impact on consumer acceptance or rejection (Shahidi et al., 1998) as well as product market price.
Skin colouration highly depends on the deposition of pigments (melanin, carotenoids, pteridines and purines) in the different types of cromatophors and light reflections. This deposition is controlled by both internal and external parameters. For instance, fish colouration is altered under certain environmental conditions, physical changes, or stress. Up to 23 carotenoids have been found in fish tissue, including astaxanthin (3,3'- dihydroxy-ß,ß-carotene-4,4'-dione) (AX), canthaxanthin (ß,ß-carotene-4,4'-dione) (CX), ß-carotene, and lutein, which is the most abundant (Czeczuga et al., 1991). But since fish, like other vertebrates, are unable to synthesise carotenoids de novo (Goodwin, 1984), skin colouration is directly dependent on dietary carotenoids. More than 600 naturally occurring carotenoids are now known, all being derived from the same basic C40 isoprenoid skeleton (Britton et al., 1995). They owe their colour to the absorption of light by a feature of their molecular structure that is known as the chromophore. Natural production of carotenoids occurs mainly in the photosynthetic tissues of plants and algae, their presence being masked by the green chlorophyll.
Carotenoids later accumulate in roots, fruit, or flowers. Both phototrophic and non-phototrophic bacteria, moulds and yeasts are also sources of carotenoids. Annual natural production of carotenoids is estimated to be about 100 million tons, of which fucoxanthin (the main carotenoid in brown seaweeds) contributes about 10 million tons, and peridinin (from dinoflagellates) contributes even more (Britton et al., 1995).
Distribution of carotenoids in fish tissues
Carotenoid concentration and distribution among the different fish tissues seems to depend on the species, as well as the life cycle. For instance, rainbow trout fingerlings accumulate carotenoids in the skin, whereas later, during periods of fast growth, carotenoids accumulate mainly in the muscle (Czeczuga et al., 1991). Salmonids are quite unique among fish in their ability to deposit substantial amounts of dietary carotenoids in their muscle tissues. AX is the major carotenoid of wild salmonids, as well as in cultured salmon fed AX. However, AX is not detected in salmon fed an AX-free diet. CX, together with trace amounts of AX, is the main pigment found in salmon fed CX (Sheenhan et al., 1998). Flesh pigment deposition of astaxanthin or canthaxanthin in Atlantic salmon is linearly related to feed dose for both carotenoids (Baker et al., 2002).
Carotenoids are also deposited in several types of tissues and organs such as skin, flesh, gonads, intestine, kidney, liver, and only in very small amounts in the brain. In Artic charr (Salvenilus alpinus L.) fed CX for 24 wks, fish flesh is the main tissue where carotenoids are stored, followed by skin, liver and gonads. CX was the main carotenoid deposited in the flesh, which also contained lutein and some reductive metabolites of CX, namely echineone and 4'-hydroxy-echineone, suggesting the reductive metabolism of CX. ß-carotene, the final product of CX reduction, was only present in the skin, together with isocryptoxanthin, echineone, CX, and lutein esters.
Hence, geometrical and optical isomers of AX are distributed selectively in different tissues of fish. Factors other than molecular-weight-dependent diffusion rates seem to govern the discrimination between carotenoid isomers. A discriminatory mechanism for intracellular translocation of different astaxanthin isomers from the site of uptake until incorporation into chylomicrons has been suggested by several investigators.
There is an uneven distribution of colour in red porgy skin, the front lateral zone exhibiting a more reddish hue and higher chroma values, followed by the caudal and finally dorsal zone. These results suggest a progressive deposition of astaxanthin in these three body zones, since colouration and the concentration of carotenoids are significantly related (Bjerkeng, 2000). Uneven colour distribution has also been found in salmonid muscle, where a longitudinal variation in carotenoid content and red colour was found, with more astaxanthin deposited in the caudal region than in the anterior region (Bjerkeng, 2000).
Time of seawater transfer of Atlantic salmon smolts also has a significant effect on carotenoid accumulation. However, in Artic charr, sex and maturity status do not have an apparent effect on the relative composition of skin carotenoids (Bjerkeng, 2000). Neither do stress conditions, such as stocking density, seem to affect the accumulation of carotenoids in flesh.
Carotenoid functions in fish
Carotenoids may have various biological effects in fish such as supplying provitamin A, antioxidation (involving lipid peroxidation) or inmunoenhancement. In other vertebrates they also inhibit mutagenesis and transformation and pre-malignant lesions. In addition, carotenoids have been proven to decrease the risk of cataract formation, several types of cancers, and cardiovascular disease.
For example, feeding rainbow trout marine algae Dunaliella salina (rich in ß-carotene) and red yeast Phaffia rhodozyma (rich in AX) improved several humoral factors, such as serum alternative complement activity and serum lysozyme activity, as well as cellular responses, such as phagocytic rate (Amar et al., 2003).
Carotenoids also play an important role in fish reproduction (Izquierdo et al., 2001). In yellowtail (Seriola quinqueradiata), dietary astaxanthin, supplemented at about 30 mg/kg, is a determining factor for good egg quality, whereas in striped jack, although its eggs do not contain carotenoids, dietary astaxanthin added at 10 mg/kg to the diet markedly increases fecundity (Watanabe and Vasallo-Agius, 2003). Indeed, a significant interaction between astaxanthin and vitamin A has been found to be a determinant for ovarian development and spawning (Pangantihon-Kühlmann et al., 1998).
Although carotenoids are known to have a positive role in the intermediary metabolism of fish, enhancing nutrient utilisation and improving growth (Amar et al., 2001), feeding red porgy with 40 to 100 mg/kg CX, shrimp or krill meal, does not affect fish growth or feed utilization (Chebbaki, 2001; Kalinowski et al., 2005). Similar results have been found in gilthead sea bream (Sparus aurata) fed different carotenoids (Gomes et al., 2002). In rainbow trout, despite growth to 120 to 400 g, fish were not affected by an AX-supplemented diet. However, growth was significantly improved when younger fish (6-25 g) were fed AX until 400 g (Nickell and Bromage, 1998). These results suggest that growth enhancement by dietary carotenoids is more efficient in younger fish or over long-term feeding.
Regarding the effect of supplementation time on growth, the inclusion of shrimp shell meal in red porgy diets, as a source of esterified astaxanthin, enhanced growth, increasing as a function of time (unpublished data). For instance, growth of fish fed 40 mg/kg esterified AX for 180 days attained higher growth results than fish fed for 120 days, and fish fed 120 days attained better growth results than fish fed for 60 days. Nickell and Bromage (1998) found better growth results when rainbow trout diets were supplemented for long periods. Nevertheless, there is controversy regarding the effect of carotenoids on growth. Some investigators have reported enhancement with carotenoid supplementation (Torrissen, 1984; Christiansen et al., 1995), whereas others have found no effect at all (Nakano et al., 1995; Nickell and Bromage, 1998; Nakano et al., 1999).
Therefore, we cannot reject the possibility that growth enhancement may be related to other components of shrimp shell meal besides carotenoids, such as certain amino acids or phospholipids. It is also interesting to note that growth and feed utilization values obtained with this species are close to those found in gilthead sea bream reared under similar experimental conditions in our facilities (unpublished data). In spite of being new species, its efficient growth and feed utilization, added to previous findings of its good adaptability to culture conditions, spontaneous spawning in captivity, and low disease and mortality rates (Kentouri et al., 1994; Kokokiris, 1998; Schuchardt et al., 2000), makes this species attractive for aquaculture diversification.
Carotenoid absorption and deposition
Absorption of carotenoids has been associated with midgut (from pylorous to hindgut) and hindgut (the posterior, folded part of the intestine) sections (Torrissen et al., 1990), but probably changes with the species since it is linked to lipid absorption (Izquierdo, 1998; Izquierdo and Henderson, 1998; Izquierdo et al., 2000). Almost no specific studies have been conducted to date concerning the mechanism of absorption of carotenoids from the intestinal lumen. Salmonids preferentially absorb and deposit more polar carotenoids, particularly AX rather than CX and zeaxanthin or ß-carotene (Osterlie et al., 1999); but a larger amount of carotenoid is apparently ingested than is retained in the fish, suggesting that some carotenoids are broken down in the digestive tract (Storebakken et al., 1987), possibly during the formation of vitamin A or via oxidation (Torrissen et al., 1990).
Canthaxanthin-supplemented diets affected red porgy skin colouration giving a yellowish hue and atypical chroma values. Hence, despite the fact that increased intake of dietary carotenoids usually raises chroma values, raising CX dietary levels from 40 to 100 mg/kg actually reduced chroma values in red porgy. Similarly, in a closely related species, red sea bream (Pagrus major) fed ß-carotene or CX had reduced carotenoid levels in the integument (Lorenz, 1998). Such reduction in carotenoid deposition could be explained by a limitation in the rate of absorption (Torrissen et al., 1990).
Carotenoid absorption and deposition is affected by diet composition. Increases in dietary fat produce higher carotenoid deposition in flesh in trout and salmon (Torrissen et al., 1990), and increases in dietary polyunsaturated fatty acids (PUFA) enhance fillet carotenoid content (Bjerkeng et al., 1999). Since PUFA affect carotenoid absorption and deposition, dietary fish oil substitution by vegetable oils may markedly reduce carotenoid utilisation in fish. For instance, salmon fed soybean oil-based diets show a lower carotenoid content and redness than salmon fed fish oil (Bencze et al., 2004). In addition, esterified forms of carotenoids seem to be absorbed and accumulated far less than the unesterified forms. For instance, krill meal, containing 40 mg/kg esterified AX, gives red porgy an overall reddish skin colouration, in contrast to the lower pigmentation of fish fed CX at 40-100 mg/kg, suggesting a better utilisation of the esterified astaxanthin by this species.
Astaxanthin esters were also more efficiently utilised for deposition and skin colouration than free astaxanthin in red sea bream (Lorenz, 1998). In other species, such as Australian snapper Pagrus auratus, another fish with reddish skin colouration, the inclusion of astaxanthin in the diet is needed to attain natural colouration. However, this species can efficiently use both unesterified or esterified forms, resulting in similar AX content in its skin (Booth et al., 2004). Choosing the proper astaxanthin source and amount is of considerable importance for giving the skin an appropriate colour. In gilthead sea bream, synthetic astaxanthin and canthaxanthin or pigments from algae are efficiently absorbed, as shown by plasma carotenoid composition (Gomes et al., 2002). Both astaxanthin and canthaxanthin, either alone or in combination, have been efficiently used for muscle pigmentation in salmonids (Bjerkeng, 2000). However, astaxanthin in an esterified form seems to be less efficiently used by these species.
Astaxanthin is the most common carotenoid used for commercial salmonid pigmentation (Storebakken and No, 1992). Cultured salmon and trout are typically reared on diets containing either AX or CX. Both pigments are available in synthetic form and both are currently permitted within the EU for inclusion in feeds at up to 80 mg/kg CX and 100 mg/kg AX (EC Feed Additive Directive 70/524). Generally, feeds for maturing Atlantic salmon are supplied with 50 to 100 mg/kg of pigment for 6 to 18 months depending on the length of the final growing phase and the intended size of the fish at harvest. According to Torrissen et al. (1990), in commercial salmon farming this pigmentation is achieved by addition of either AX or CX to the diet, usually in the range of 35 to 75 mg/kg dry diet. The cost of this supplementation is substantial and can be responsible for between 10 and 15% of the total feed cost (Torrissen et al., 1990). A commercial dry diet supplemented with 50 mg of pigment/kg, and having a feed conversion of 1.5, will yield a carotenoid retention value in the range of 6 to 10%. The loss of 90% or more of dietary AX or CX is due to four main factors: 1) destruction of carotenoids during feed processing and subsequent storage, 2) feed wastage, 3) poor absorption of ingested carotenoids, and 4) metabolism of absorbed carotenoids (Torrissen et al., 1990).
SKIN COLOURATION IN RED PORGY
Dietary addition of free astaxanthin seemed to produce an undesirable black-reddish colour in red porgy skin. Carotenoid supplementation does not seem to affect red porgy skin lightness (Kalinowski et al., 2005). Skin pigmentation in vertebrates is modified by hormonal stimulation, background colour, and illumination (Rotllant et al., 2003). For instance, in Australian red snapper, skin darkness seems to be a response to the proximity of sea cages to the surface of the water and the subsequent effect of sunlight exposure on melanin concentration in the skin (Booth et al., 2004). Handling stress and methods used in killing the fish are also among the factors that negatively influence fish skin lightness (Qun Lin et al., 1998).
Red porgy skin pigmentation can be modified from a dark grey to a red-pink-silver colour by supplementing the diet with 40 mg of astaxanthin/kg diet contained in shrimp shell meal (Kalinowski et al., 2005). Among several colour zones evaluated, the front lateral zone seems to accumulate more astaxanthin, showing a more reddish colouration than the other two zones and less variability; hence, this is recommended as a control zone to evaluate the overall redness attained in the skin of this species. Nevertheless, since diet is not the only parameter that modifies skin pigmentation, its relation to environmental factors and aquaculture-related stressors should be considered in order to obtain a satisfactory colouration of red porgy.
EFFECT OF FEEDING PERIOD ON COLOURATION
Enhanced reddish hue and chroma obtained in red porgy skin by feeding carotenoids tended to be reduced after 105 days of feeding, suggesting a skin colour saturation. In agreement, an apparent colour saturation point was found in red sea bream fed a diet containing 100 mg free astaxanthin/kg for a month, with no further increase thereafter (Ito et al., 1986), whereas when fish were fed astaxanthin esters, maximum astaxanthin contents were found after two months of feeding. This saturation plateau found in fish fed carotenoids seems to depend on genetic (Torrissen and Naevdal, 1984), nutritional (Torrissen, 1984) and biological factors such as fish size and species.
Hence, feeds for maturing Atlantic salmon are supplemented with 50 to 100 mg/kg of pigment for 6 to 18 months depending on the length of the final growing phase and the intended size of the fish at harvest. The content of carotenoids in fillets of charr generally increased with duration of feeding the pigmentsupplemented diet.
Red porgy skin colouration is improved by the inclusion of carotenoids in the form of krill meal, shrimp (Pleisonika spp., Cejas et al., 2003) or shrimp shell meal (Kalinowski et al., 2005). Nevertheless, there is little information on the optimum period of supplementation with astaxanthin for red porgy to achieve a skin colouration similar to wild specimens. Supplementation time is not only important for attaining the proper pigmentation; but also for lowering the total feed and production costs, as discussed above.
Supplementing red porgy diets with 40 mg/kg esterified AX for 180 and 120 days produced a skin hue similar to that of wild specimens. Saturation degrees or chroma values attained by fish fed supplements for 180, 120 and 60 days were almost the same. These results suggest some form of biological saturation. These observations are in agreement with our previous studies using shrimp shell meal, where at day 75 chroma values reached a plateau (authors’ unpublished data). Similarly, Gomes et al. (2002) found that the concentration in skin of gilthead sea bream, a closely related species, plateaued after 3 weeks of feeding diets containing 40 mg/kg unesterified astaxanthin from Carophyll Pink. Although chroma values similar to wild specimens are acquired with only 60 days of supplementation before harvest, 120 days are needed to obtain hue values matching wild specimens.
Carotenoid supplementation for 120 days improved both hue and chroma, nevertheless, the skin lightness variable was not positively affected with 60, 120 or 180 days of supplementation. Fish fed an unsupplemented diet attained the best lightness results (Figure 1). An experiment conducted with artic charr reported a positive relationship between carotenoid concentration, redness (a*) and yellowness (b*) values, whereas lightness (L*) was negatively correlated with carotenoid concentration (Hatlen, 1998). Bjerkeng et al. (1997) also found that L* values had a negative, linear correlation with the fillet carotenoid concentration.
Not only is lightness affected negatively by carotenoid concentration, but also by other factors such as stress, handling and killing method (Qun Lin et al., 1998).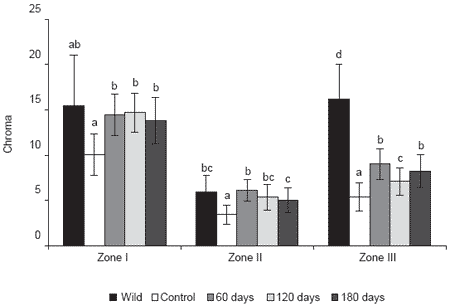
Figure 1. Skin chroma of wild red porgy and red porgy fed either a control diet (no astaxanthin addition) or a diet containing 40 mg/kg astaxanthin for 60, 120 or 180 days.
EFFECT OF STORAGE TIME ON PIGMENTATION
Storage time and conditions may also affect the skin or flesh colouration attained in fish fed different types of carotenoid sources. For instance, there was no significant difference between AX concentration of raw flesh measured before and after frozen storage (-20°C) in Atlantic salmon fed for 6 or 12 weeks (Sheehan et al., 1998). However, salmon fed CX diets showed a significant reduction in the CX in flesh after 12 weeks of frozen storage, suggesting that CX-pigmented fish should only be stored for a maximum of 6 weeks. Since less colour change occurs over time in fish that have been smoked, CX-pigmented fish are more suited for smoking. A mix of AX and CX is required to produce fish capable of undergoing frozen storage and/or smoking (Sheehan et al., 1998).
Conclusion
Supplementing Pagrus pagrus diets with 40 mg/kg esterified astaxanthin from shrimp shell meal for a period of 120 days is enough to acquire a skin colouration similar to wild red porgy. Nevertheless, to completely determine the length of supplementation for this species, other experiments should be conducted to study the loss of skin colouration post-mortem, since fish normally reach the market several days after they have been killed.
References
Amar, E.C., V. Kiron, S. Satoh and T. Watanabe. 2001. Influence of various dietary synthetic carotenoids on bio-defence mechanisms in rainbow trout, Oncorhynchus mykiss (Walbaum). Aqua. Res. 32(Suppl. 1):162-163.
Amar, I. A. Aserin and N. Garti. 2003. Solubilization patters of lutein and lutein esters in food grade nonionic microemulsions. J. Agric. Food Chem. 51(16):4775- 4781.
Baker, R.T.M., A.M. Pfeiffer, J. Schöner and L. Lemmon-Smith. 2002. Pigmenting efficacy of astaxanthin and canthaxanthin in fresh-water reared Atlantic salmon, Salmo salar. Anim. Feed Sci. Technol. 99(1/4):97-106.
Bencze Røra, A.M., S. Birkeland, L. Hultmann, T. Rustad, T. Skåra and B. Bjerkeng. 2004. Quality characteristics of farmed Atlantic salmon (Salmo salar) fed diets high in soybean or fish oil as affected by cold-smoking temperature. Lebensmittel Wissenschaft und Technologie (in press).
Bjerkeng, B. 2000. Carotenoid pigmentation of salmonid fishes – recent progress. In: Avances en Nutrición Acuícola V. Memorias del V Simposium Internacional de Nutrición Acuícola (L.E. Cruz– Suárez, D. Rique-Marie, M. Tapia-Salazar, M.A. Overa-Novoa y R. Civera-Cerecedo, eds). 19-22 Noviembre, 2000. Mérida, Yucatán.
Bjerkeng, B., S. Refstie, K.T. Fjalestad, T. Storebakken, M. Roedbotten and A.J. Roem. 1997. Quality parameters of the flesh of Atlantic salmon (Salmo salar) as affected by dietary fat content and full-fat soybean meal as a partial substitute for fish meal in the diet. Aquaculture 147(3-4):295-307.
Bjenkeng, B., B. Hatlen and E. Wathne. 1999. Deposition of astaxanthin in fillets of Atlantic salmon (Salmo salar) fed diets with herring, capelin, sandeel or Peruvian high PUFA oils. 1999. Aquaculture 180(3-4):307-309.
Booth, M., R. Warner-Smith, G. Allan and B. Glencross. 2004. Effects of dietary astaxanthin source and light manipulation on the skin colour of Australian snapper, Pagrus auratus (Bloch & Schneider, 1801). Aqua. Res. 35:458-464.
Britton, G., S. Liaaen-Jensen and H. Pfander. 1995. Carotenoids, Vol. 1A, 1B. Birkhäuser Velag, Berlin, Germany.
Cejas, J., E. Almansa, N. Tejera, S. Jerez, A. Bolaños and A. Lorenzo. 2003. Effect of dietary supplementation with shrimp on skin pigmentation and lipid composition of red porgy (Pagrus pagrus) alevins. Aquaculture 218:457-469.
Chebbaki, K. 2001. Efecto de la nutrición sobre la coloración de la piel y la calidad del filete en bocinegro (pagrus pagrus). Master Thesis. University of Las Palmas de Gran Canaria.
Christiansen, R., J. Glette, Ø. Lie, O.J. Torrissen and R. Waagbø. 1995. Antioxidant status and immunity in Atlantic salmon, Salmo salar, L., fed semi-purified diets with and without astaxanthin supplementation. J. Fish Dis. 18:317-328.
Czeczuga, B., S. Stenross, S.N. Christensen and T. Ahti. 1991. Variability of carotenoid composition in some species of the lichen genera Cladonia and Cladina. Annales-Botanici-Fennici. 28(2):123-130.
Gomes, E., J. Dias, P. Silva, L. Valente, J. Empis, J.B. Gouveia and A. Young. 2002. Utilization of natural and synthetic sources of carotenoids in the skin pigmentation of gilthead sea bream (Sparus aurata). Eur. Food Res. Technol. 214:287-293.
Goodwin, T.W. 1984. Tunicates and Fish. In: The Biochemistry of the Carotenoids. Vol. II, Chapman and Hall, London, pp. 224.
Hatlen, B., M. Jobling and B. Bjerkeng. 1998. Relationships between carotenoid concentration and colour of fillets of Arctic charr, Salvelinus alpinus (L.), fed astaxanthin. Aqua. Res. 29(3):191-202.
Hernandez-Cruz, C.M., H. Fernandez-Palacios and J.E. Fernandez-Palacios. 1990. Estudio preliminar del desarrollo embrionario y larvario del bocinegro, Pagrus pagrus (Pisces: Sparidae) en cultivo. Vieraea 19:215-224.
Hernandez-Cruz, C.M., M. Salhi, M. Bessonart, M.S. Izquierdo, M.M. Gonzalez and H. Fernandez-Palacios. 1999. Rearing techniques for red porgy (Pagrus pagrus) during larval development. Aquaculture 179:489-497.
Ito, Y., T. Kamata and M. Sameshima. 1986. Studies on the improvement of body colour of red sea bream Pagrus major by astaxanthin and astaxanthin dipalmitate. Suisanzoshoku 34:77-80.
Izquierdo, M.S., J. Socorro, L. Aranzamendi and C.M. Hernandez-Cruz. 2000. Recent advances in lipid nutrition in fish larvae. Fish Physiol. Biochem. 22:97- 107.
Izquierdo, M.S. 1998. Digestión, absorción, transporte y utilización nutritiva de los lípidos dietéticos en peces marinos. Act. IV Simposium Internacional De Nutrición Acuícola, La Paz, México, pp. 25-34.
Izquierdo, M.S. and R.J. Henderson. 1998. The determination of lipase and phospholipase activities in gut contents of turbot (Scophthalmus maximus) by fluorescence-based assays. Fish Physiol. Biochem. 19:153-162.
Izquierdo, M.S., H. Fernandez-Palacios and A.G.J. Tacon. 2001. Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 197:25-42.
Kalinowski, T., L.E. Robaina, H. Fernandez-Palacios, D. Schuschardt and M.S. Izquierdo. 2005. Effect of different carotenoid sources and their dietary levels on red porgy (Pagrus pagrus) growth and skin colour. Aquaculture 244:223-231.
Kentouri, M., D. O´Neil, P. Divanach and G. Charalambakis. 1994. A study of the quantitative water requirements of red porgies Pagrus pagrus L. (Pisces: Sparidae), during early growing under self feeding conditions. Aqua. Fish. Man. 25:741-752.
Kentouri, M., M. Pavlidis, N. Papandroulakis and P. Divanach. 1995. Culture of red porgy, Pagrus pagrus, in Crete. Present knowledge, problems and perspectives. Cah. Options Mediterr. 16:65-78.
Kokokiris, L. 1998. The reproductive cycle and hermaphrodite pattern in Pagrus pagrus. Ph.D Thesis, University of Crete, pp. 219.
Lorenz, T.R. 1998. A review of astaxanthin as a carotenoid and vitamin source for sea bream. Naturerose Technical Bulletin N 052. Cyanotech Corporation, Hawaii, USA.
Nakano, T., M. Tosa and M. Takeuchi. 1995. Improvement of biochemical features in fish health by red yeast and synthetic astaxanthin. J. Agric. Food Chem. 43:1570-1573.
Nakano, T., T. Kanmuri, M. Sato and M. Takeuchi. 1999. Effect of astaxanthin rich red yeast (Phaffia rohodozyma) on oxidative stress in rainbow trout. Biochim. Biophys. Acta 1426:119-125.
Nickell, D.C. and N.R. Bromage. 1998. The effect of timing and duration of feeding astaxanthin on the development and variation of fillet colour and efficiency of pigmentation in rainbow trout (Oncorhynchus mykiss). Aquaculture 169:233-246.
Osterlie, M., B. Bjerkeng and S. Liaaen-Jensen. 1999. Accumulation of astaxanthin all-E, 9Z and 13Z geometrical isomers and 3 and 3' RS optical isomers in rainbow trout (Oncorhynchus mykiss) is selective. J. Nutr. 129(2):391-398.
Pangantihon-Kühlmann, M.P., O. Millamena and Y. Chern. 1998. Effect of dietary astaxanthin and vitamin A on the reproductive performance of Penaeus monodon broodstock. Aqua. Living Resour. 11(6):403-409.
Qun Lin, M., H. Ushio, T. Ohshima, H. Yamanaka and C. Koizumi. 1998. Skin color control of the red sea bream (Pagrus major). Lebensm.-Wiss.u.-Technol. 31:27-32.
Rotllant, J., L. Tort, D. Montero, M. Pavlidis, M. Martinez, S.E. Wenderlaar Bonga and P.H.M. Balm. 2003. Background colour influence on the stress response in cultured red porgy Pagrus pagrus. Aquaculture 223:129-139.
Schuchardt, D., J.M. Vergara, L. Robaina and D. Montero. 2000. The effects of varying dietary protein and lipid levels on growth, feed efficiency, protein utilization and body composition of red porgy fingerlings. The 9th Intern. Symp. on Nutrition and Feeding in Fish 2000, Miyasaki, JAPAN, p. 91.
Shahidi, F., A. Metusalach and J.A. Brown. 1998. Carotenoid pigments in seafoods and aquaculture. Crit. Rev. Food Sci. Nutr. 38(1):1-67.
Sheehan, E.M., T.P. O´Connor, P.J.A. Sheehy, D.J. Buckley and R. FitzGerald. 1998. Stability of astaxanthin and canthaxanthin in raw and smoked Atlantic salmon (Salmo salar) during frozen storage. Food Chem. 63(3):313-317.
Stephanou, D., G. Georgiou and E. Shourkri. 1995. Reproduction and larval rearing of the common sea bream (Pagrus pagrus), an experimental culture. Cah, Options Mediterr. 16:79-87.
Storebakken, T., P. Foss, K. Schiedt, E. Austreng, S. Liaaen-Jensen and U. Manz. 1987. Carotenoids in diets for salmonids 4. Pigmentation of Atlantic salmon with astaxanthin, astaxanthin dipalmitate and canthaxanthin. Aquaculture 65(3-4):279-292.
Storebakken, T. and H.K. No. 1992. Pigmentation of rainbow trout. Aquaculture 100:209-229.
Torrissen, O.J. 1984. Pigmentation of salmonids. Effects of carotenoids in eggs and start feeding diet on survival and growth rate. Aquaculture 43:185-193.
Torrissen, I.J. and G. Naevdal. 1984. Pigmentation of salmonids – genetic variation in carotenoid deposition in rainbow trout (Salmo gairdneri). 1984. Aquaculture 38(1):59-66.
Torrissen, O.J., R.W. Hardy, K.D. Shearer, T.M. Scott and E.E. Stone. 1990. Effects of dietary canthaxanthin level and lipid level on apperent digestibility coefficients for canthaxanthin in rainbow trout (Oncorhynchus mykiss). Aquaculture 88:351-362.
Watanabe, T. and R. Vassallo-Agius. 2003. Broodstock nutrition research on marine finfish in Japan. Aquaculture 227:35-61.
Authors: M.S. IZQUIERDO1, C.T. KALINOWSKI1, S. THONGROD2 and L. ROBAINA1
1 Grupo de Investigación en Acuicultura, ULPGC & ICCM, Las Palmas, Canary Islands, Spain
2 Coastal Aquatic Feed Research Institute, Sriracha, Chonburi Province, Thailand



.jpg&w=3840&q=75)