INTRODUCTION
Grouper fish are genera of the subfamily Epinephelinae of the family Serranidae, in the order Perciformes (Cornish and Harmelin-Vivien, 2004). The common name grouper is usually given to fish in one of two large genera: Epinephelus and Mycteroperca (Cornish and Harmelin-Vivien, 2004). Despite the fact that Grouper fish are variable in size, they can be moderately large with maximum lengths over a meter and weights over 65 kg in some sporadic cases (Jory and Iverson, 1989). It is a protogynous hermaphrodite, changing from female to male at large size. Females in Southern Mediterranean areas like Tunisia reached sexual maturity at age 5, while sex reversal of females to males takes place between the 9th and 16th years with a maximum at the12th year (Chauvet, 1981; Bannerot, 1984). Groupers are predator type of fish that swallow prey rather than cutting pieces off it. They possess few teeth on the edges of their jaws, followed by strong crushing plates inside the gill pharyngeal cavity. This category of fishes is carnivorous in nature where they prey on fish, octopus, crab, lobster and dead carcasses in the lack of their natural food prey (Heemstra and Randall, 1993). Grouper fish are that kind of predators that lie in wait to surprise their prey, rather than chasing in open water (Heemstra and Randall, 1993).
More than 14 species of grouper are widely distributed along vast areas of the world s marine habitats, which include the Atlantic Ocean, Mediterranean Sea, Red Sea, Indian Ocean and Pacific Ocean (Heemstra and Randall, 1993). Dusky grouper (Epinephelus marginatus) is one of the grouper fishes that are native to the Mediterranean coastal zones (Aronov, 2002). Their range extends from France, Spain, Moroco, Tunisia, Egypt and Israel Mediterranean coastal shores (Cornish and Harmelin-Vivien, 2004). Dusky groupers are much more abundant in the southern part of the Mediterranean than in the north. However, a decline in populations has been observed in Tunisia and southern Spain due to overfishing (Cornish and Harmelin-Vivien, 2004). Overexploitation from commercial fishing is the primary threat. The slow growth rate and the complex reproductive style of E. marginatus complicate its inability to withstand high fishing pressure (Fennessy, 1998).
Over the last two decades, aquaculture has proliferated in the coastal zones of south Mediterranean countries with consequent increase in the economical influx to these nations. Once counted as an environmentally safe practice, aquaculture is currently considered as a potential source of pollution to the marine environment (Findlay et al., 1995). Organic enrichment of the seabed is the most critically posed impact of cage mariculture (Gowen and Bradbury, 1987; Iwama, 1991). A small proportion of the carbon supplied to the fish via the feed is retrieved through harvest, whereas a considerable amount reaches the seabed, either as wasted food pellets or as fecal excretions. In salmonid cage farming, 29% of carbon (Hall et al., 1991), 23% of nitrogen (Hall et al., 1991) and 47-54% of phosphorus (Holby and Hall, 1991) may be lost in particulate form and end up on the sea bottom. It is also likely that the high population densities together with the presence of high levels of excreted carbon and nitrogen compounds in the re-circulated water in hatcheries contribute to the growth, establishment and multiplication of aquatic fastidious organisms such as mycobacterium (Ashburner, 1977; Hedrick et al., 1987).
Mycobacteriosis, caused by aquatic Mycobacterium species, is a common disease in a wide variety of fish species world-wide. Genus Mycobacterium is classified under the family Mycobacteriaceae , which consists of 54 species at present, although it seems likely that some still remain to be classified (Master,1995). In aquatic environment, three species of Mycobacterium, M. marinum, M. fortuitum and M. chelonae, have often been reported as the cause of mycobacteriosis in wide variety of aquatic species (Nigrelli and Vogel, 1963; Ross, 1970; Giavenni and Finazzi, 1980; Colorni, 1992; Puttinaowarat, 1999; Jacobs et al., 2009). All three mycobacteria are also potential human pathogens (Paul and Gulick, 1993; Guay, 1996; Kullavanijaya, 1999). Among aquatic mycobacteria, M. marinum is the most prevalent species in tropical freshwater and marine fishes (van Duijn, 1981).
Mycobacteriosis of fish can be possibly transmitted in the aquatic environment by ingestion of contaminated food or aquatic debris, although bacterial invasion through damaged skin or gill tissue may also be possible (Frerichs, 1993; Hawke, 2000; Jacobs et al., 2009). Mariculture of translocated stocks or invasive species increases the risk of introduction of disease agents to new areas, with potentially serious consequences to native fishes (McVicar, 1975). Introduced pathogens originating in cage-farmed fish may establish self-shedding reservoirs in cohabiting wild fish populations (Munro et al., 1983; Treasurer and Cox, 1991). One of the most serious disputes against aquaculture is that accidental release of fish, or discharge of untreated water in which they were kept, may propagate infections in the natural environment (Coutant, 1998). Regionally, fish mycobacteriosis has been one of the most devastating outbreaks associated with the Israeli marine cage culture in recent years. The disease was first diagnosed in Eilat (Red Sea) in 1990 in cage cultured sea bass, Dicentrarchus labrax (Colorni, 1992), and the causative agent, M. marinum, has been detected in several marine fish species since then (Diamant et al., 2000). High percentage of wild rabbitfish caught between marine cages in Eilat was found to be affected by the same strain of M. marinum. Transmission of M. marinum between fish and amphibians through co-cultivation or exposure to M. marinum contaminated water has also been reported (Clark and Shepard, 1963).
Clinical signs of mycobacteriosis may include skin discoloration, inappetence, lethargy, abnormal swimming behavior, segregation, cutaneous ulcerations or erosions, ascitis, reduced growth, and exophthalmia (Wood and Ordal, 1958; Jacobs et al., 2009). Internally, the disease usually characterized by the presence of multiple granulomatous nodules scattered within the internal organs including liver, spleen, kidneys and intestine with or without adhesions (Austin and Austin, 2007; Frerichs, 1993; Noga, 1995). Traditional diagnosis of this pathogen is mainly based on recovery of the pathogen on culture medium followed by a panel of differential biochemical tests (Kent and Kubica, 1985; Austin and Austin, 2007; Jacobs et al., 2009) for final presumptive identification. This classical method which might takes up to several weeks, sometimes fails to identify M. marinum conclusively (Aronson 1926; Abalain-Colloc et al., 2003; Austin and Austin, 2007). Tissue staining techniques can give a primary inference to the granulomatous lesions encountered in the affected tissue (Nyka & O’ Neill, 1970; Gauthier et al., 2003). Recent advances in molecular biology have triggered a quick diagnosis of mycobacterial isolates to the species level using nucleic acid based molecular techniques (Knibb et al., 1993; Ranger et al., 2006; Yip et al., 2007).
The zoonotic capability of M. marinum has been documented in large number of research articles. M. marinum lives saprophytically in warm aquatic environments and appears unusual in being not only fish pathogen but also a human pathogen, causing chronic skin ulcerations called swimming-pool granulomas. The case can be complicated in AIDS, IBIOLA virus patients and other immunosuppressive diseases where the disease takes a systemic form (Enzensberger et al., 2002; Lahey, 2003).
The current study presents a unique record of Mycobacteriosis as one of the most important emerging diseases among grouper populations at the coastal south Mediterranean zones of Egypt.
MATERIALS AND METHODS
Sampling
A total of 5 representative dusky grouper fishes were collected during the event of mass mortalities among the grouper population from the Saloum bay and rocky coastal zones of Marsa Matrouh province, Egypt. Fishes were found apparently dying between the rocky communities at the shallow coastal lagoons of Marsa Matrouh Mediterranean province. The groupers mortalities were at their peak during late summer and early fall of 2008. Fish were kept on crushed ice inside well insulated ice box till transported to the Fish Disease and Management Laboratory (FDML) at Cairo University few hours after the event of mortalities.
Sample processing
Collected fish were euthanized using an overdose of MS222 (Tricaine Methane Sulfonate – Finquel- Argent Chemical Laboratories, Washington) in sea water then clinically examined for any external abnormalities before being dissected. Fish were dissected under complete aseptic condition then pooled chunks from spleen, liver and kidney were cut into very small pieces in a sterile plastic Petri dish then homogenized in Hanks Balanced Salt Solution (HBSS , 1:4 w/v) (Sigma Chemical Co, St. Louis, MO, USA). Equal volume of Na OH 4 % was added to each pooled homogenate sample. The mixture was further neutralized by adding phosphate buffered saline (pH=7) then centrifuged at 3000 rpm for 30 min (Isenberg, 1998).
Bacteriological Examination
The resulted pellets were re-suspended in HBSS solution then 100 μl of the suspension were streaked onto Lowenstein - Jensen media slants supplemented with 5% Sodium Chloride (BBL-Becton Dickinson- Franklin Lakes, NJ, and USA). Inoculated slants were incubated at 28 ºC and 37 ºC for 14 days. Slants were inspected on weekly base for any bacterial colonial growths. Colonies were examined for acid-alcohol fastness by the Ziehl - Neelsen technique. The bacterium was identified by its rate of growth, colonial morphology, pigmentation at dark and light, and biochemical properties. The bacterium was tested for growth on MacConkey agar plate, salt tolerance on Dorset Egg media supplemented with 0, 1, 3, and 5% NaCl, Tween 80 hydrolysis, urease, heat-stable catalases (68°C), niacin production, tellurite reduction, nitrate, and pyrazinamide utilization. The identification scheme of the retrieved isolates was adopted from Austin and Austin (2007) and Holmes et al. (1999).
Histopathology
Tissues sample were fixed in 10% neutral buffered formalin solution, and were then processed and embedded in paraffin. Five-micron sections of tissue were stained with hematoxylin and eosin (H & E) using methods described by Bancroft et al. (1996). Selected sections were stained using Periodic Acid Chief (PAS) and Ziehl Neelsen’s method for acid-fast bacteria. Random sections of H&E stained liver tissue were examined at 100 - 400 X for the presence and number of mycobacterial granulomas.
Water analysis
Water samples from (1- 4 meter) depths were collected from the Saloum bay and rocky coastal zones of Marsa Matrouh province during the event of mortalities. The water samples were analyzed for any changes in the measures of water pH, temperature, dissolved oxygen and toxic ammonia. Water temperature and pH were measured using a waterproof digital combo pH meter & thermometer (HI 98127 (pHep 4) -Hanna instruments Inc., RI, USA). Dissolved oxygen (DO) concentration were measured using a digital dissolved oxygen meter (HI 9142 - Hanna instruments Inc., RI, USA). Total ammonia nitrogen (TAN mg/L) was determined following the method described by Chattopadhyay (1998).
RESULTS
During our investigatory visit to the site of mass mortalities, it has been noticed that hundreds of grouper fishes were found dead on the water surface as well as in the lagoons between coastal rocks. Clinical examination of the collected moribund groupers revealed the presence of number of external clinical abnormalities including, skin discoloration, abnormal, irregular skin ulcerations at the isthmus and lateral sides of the fish, apparent exophthalmia and mild ascitis. Internal examination revealed the presence of different sized granulomatous nodules scattered within the liver, spleen. Adhesion of the internal organs together with the mesentery has been noticed in two of the affected fishes.
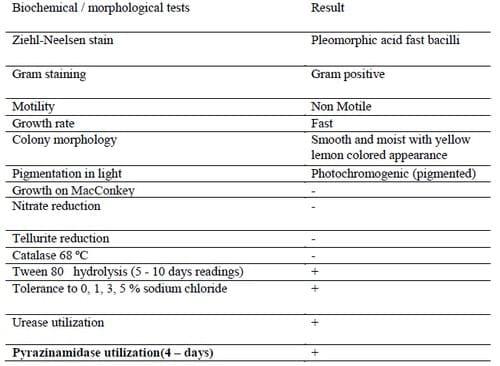
Bacterial isolates retrieved from the pooled tissue samples for each individual of the 5 groupers after 14 incubation days on Lowenstein - Jensen slants were presumptively identified as M. marinum using both cultural characteristics and conventional biochemical tests (Table 1).The retrieved isolates were aerobic, non-spore-forming, non motile, gram-positive, acid-fast bacilli. All isolates were fast growers with yellow lemon like photochromogenic smooth colonies on Lowenstein - Jensen slants. The conventional biochemical testing for the retrieved isolates revealed the inability for growth on MacConkey agar plate, ability to grow on Dorset Egg media supplemented with 0, 1, 3, and 5% NaCl. Isolates were negative for heat-stable catalases (68°C), tellurite and nitrate reduction while positive for Tween 80 hydrolysis, urease and pyrazinamide utilization.
Table 1. Morpho-chemical tests result for the retrieved M. marinum suspect isolates.
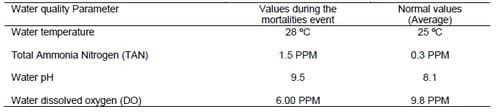
Table 2. Water quality measures during the mass mortalities event compared to the average normal values.
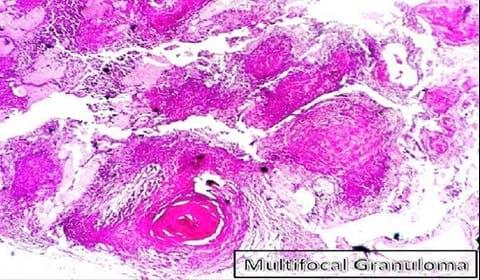
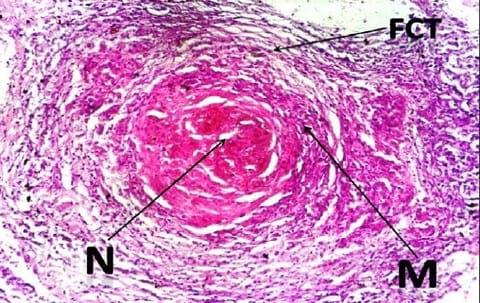
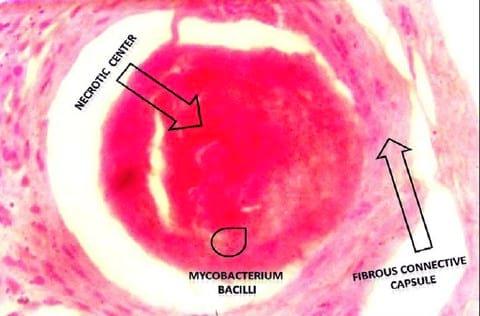
The histopathological examination of the H&E stained slides of collected groupers revealed the presence of different stages of multiple focal granulomatous reactions. Depending on the stage of the granuloma formation, two types of granulomas were detected. The detected early stages of granuloma were characterized by the presence of central inflammatory cells surrounded by outer fibrous connective tissue capsule while the older stages showed central necrosis surrounded by layers of different mononuclear cells and few giant cells (Fig.1). The entire reaction was encircled by an outer layer of fibrous connective tissue capsule (Fig. 2). Both stages of granulomas showed no evidence of calcification (Fig. 2 - 3).
Figure 1
Figure 2
Figure 3
Examination of the Ziehl Neelsen stained slides from the liver of the collected groupers revealed the presence of red acid fast pleomorphic bacilli within the core structure of the multifocal granulomas (Fig. 3).
Water quality parameters of the samples collected during the emergent event of grouper mortalities including water temperature, pH, total ammonia nitrogen (TAN) and dissolved oxygen were determined. The determined water quality measures were compared to the average normal values that were routinely measured by authorities for the same coastal areas prior to the event of mass mortalities. Abrupt increase in temperature from (25 ºC to 28 ºC), pH from (8.1to 9.5), total ammonia (TAN) from (0.3 to 1.5 PPM) and sharp decrease in dissolved oxygen from (9.8 to 6 PPM) were the environmental stimulus that initiated and boosted the infection (Table 2).
DISCUSSION
Mass fish kills in the natural waters are recurrent syndromes that utilize the death of large number of fishes within limited time and throughout a specified water basin. These deaths might include certain species of fishes or extend to more than one species sharing the same geographical location and same food chain. In our case, only grouper fishes have reflected the emergent case of mortalities. This could be linked to the unique behavioral pattern of groupers in which they swim, hide, predate, group and socialize at the water lagoons between the rocks. Those groupers can prey on dead carcasses in the lack of their natural food prey (Heemstra and Randall, 1993). The moderately static water lagoons between the rocks of Saloum Bay and its closer Marsa Matrouh coastal zones could be a natural reservoir for many environmentally hazardous wastes such as sewage, industrial effluents, shipping, yachting and anthropogenic activities . Those hazards represent a vulnerable source of infection and challenge to the immune system of groupers which consequently potentiate the occurrence or resurgence of disease outbreaks which simulates the mycobacterioisis outbreaks that happened at Chesapeake Bay in 1997 (Vogelbein et al., 1998).
The poor nature of the food web in this zone of the Mediterranean water complicated with long term environmental pollution and abrupt turn down of water quality during the hot season of the late summer and early fall were suggested to be the most triggering factors behind the initiation of the event of mass mortalities. This assumption coincides with Rhodes et al. (2004) who concluded that elevated temperature or reduced food web may also be necessary for development of fish disease outbreaks like that reported in our current study.
The cohabitation is another implementing factor in the initiation and spread of infections. The cohabitation between the same fish under the same grouper populations and with other wild species such as water reptiles, aquatic amphibians as well as aquatic migratory birds could have played a critical role in the emergence of mycobacteriosis as a disease that is new to such pristine area of the world (Belas et al., 1995; Inglis et al., 1993; McVicar, 1975; Munro et al., 1983; Treasurer and Cox, 1991). It is also likely that cohabitation between different aquatic species in the same spatial area together with the presence of high levels of excreted carbon and nitrogen compounds as organic wastes from marine cages in the neighborhood might highly contributed to the growth, establishment and multiplication of aquatic fastidious organisms such as mycobacterium (Ashburner, 1977; Hedrick et al., 1987).
The clinical examination and post mortum findings of the collected dusky groupers coincided with those reported for typical cases of mycobacteriosis in grouper and other fishes in which the granulomatous nodules were numerous within different internal organs (Wood and Ordal, 1958; Frerichs, 1993; Noga, 1995; Austin and Austin, 2007; Jacobs et al., 2009). Further, adhesions of the internal organs together with the mesentry were noticed in two of the cases. Similar findings were previously presented in several reports dealing with aquatic animal mycobacteriosis throughout the past few decades (Noga, 1995; Austin and Austin, 2007).
The results of the performed panel of growth and biochemical tests for the retrieved isolates were in complete accordance with those described for M. marinum in numerous literatures (Kent and Kubica, 1985; Tortoli, 2003; Austin and Austin, 2007; Jacobs et al., 2009). The fast growth on Lowenstein - Jensen slants after a period of 14 days incubation at 28 ºC, smooth yellow lemon colored appearance of the colonies, photochromogenicity in absence of light, inability of growth on MacConkey agar plate, salt tolerance on Dorset Egg media supplemented with 0, 1, 3, and 5% NaCl is conformant with M. marinum growth profile reported by Kent and Kubica (1985); Master (1995); Austin and Austin (2007). The biochemical profile of the retrieved isolates including the hydrolysis of tween 80; urease and pyrazinamide utilization; negative nitrate and tellurite reduction with catalase test (68 ºC) negative runs parallel to the organism profile reported by Holmes et al. (1999) and Austin and Austin (2007).
The histopathological examination of the H&E stained slides of collected groupers revealed the presence of different stages of multiple focal granulomatous reactions (Fig.1) which suggest different stages of infection due to persistent existence of the invasive pathogen in different lag phases of growth within the natural aquatic environment surrounding the fish. This assumption is strengthened by Faisal and Eissa (2009) conclusion about the existence of more than one stage of infection within the same population and same fish when exposed to natural infection of the obligate intracellular Renibacterium salmoninarum (R. salmoninarum). In fact, M. marinum and R. salmoninarum are close members of the order actinomycetals, which could give a clue about the nature and sequential pathogenesis of granuloma associated with both organisms.
The characteristic non-calcified nature of the formed granulomas (Fig. 2-3) within the liver tissue is conformant with the granulomatous reaction reported throughout the piscine mycobacteriosis literatures (Bruno et al., 1998; Wolke and Stroud, 1978; Wolf and Smith, 1999; Heckert et al., 2001). The presence of scanty number of giant cells was consistent with those reported in most of mycobacteriosis literatures (Wolke and Stroud, 1978; Wolf and Smith, 1999; Heckert et al., 2001). The presence of acid fast bacilli within the center of the soft granulomatous lesions in liver (Fig.3) coincide with previous descriptions of Zeihl Neelsen stained granulomas in numerous publications (Bruno et al., 1998; Wolke and Stroud, 1978; Wolf and Smith, 1999; Heckert et al., 2001).Water quality has strong spatial heterogeneity and temporal flux, and these conditions could exacerbate both bacterial proliferation and host susceptibility (Kane et al., 2007). Water quality measures reported in our study were moderately deteriorated if compared to the normal measures for the same water basin before the occurrence of the mass mortality event (Table 2).
This results were supported by the fact that certain water quality criteria, including those associated with degraded habitats, fluctuated high or low pH, and higher organic content, have been reported to foster the growth of environmental mycobacteria (Falkinham et al., 2004). Other factors, associated with abrupt seasonal surge of water temperature, biofilms, and even water dynamics associated with global warming, would support the flare up of environmental pathogens including aquatic mycobacteria (Harvell et al., 1999; Falkinham et al., 2004). Possible industrial and organic contamination, algal blooms, and low dissolved oxygen levels could have served as environmental stimuli for the eruption of the currently reported emerging M. marinum infection in dusky groupers of Saloum as well as Marsa Matrouh coastal zones. The analogous similarity of the aquatic environment as well as the nature of environmental degradation of the Egyptian Marsa Matrouh and Saloum bay to that of American Chesapeake bay might give a clue to the spatial environmental dynamics behind the emergence of fish mycobacteriosis outbreaks in both aquatic environments (Hall et al., 2002).
To sum up, the deteriorated environmental paradigm of the currently studied water basin together with the natural behavior of the investigated fish were highly incriminated as the main stimulus behind the emergent eruption of M. marinum outbreaks among the dusky grouper (Epinephlus marginatus) in the current study.
ACKNOWLEDGMENTS
We would like to express our deep gratitude to the officials of General Organization of Fish Wealth Development, Marsah Matrouh fishermen, Colleagues at the Department of Fish Diseases and Department of Microbiology, Faculty of Veterinary Medicine at Cairo University. We are deeply indebted to all of them for being so supportive during the course of fish sampling, sample processing and facilitating microbiological procedures throughout the entire study.
REFERENCES
ABALAIN-COLLOC M.L., D. GUILLERM, M. SALAUN, S. GOURIOU, V. VINCENT, B. PICARD .2003. Mycobacterium szulgai isolated from a patient, a tropical fish and aquarium water. Eur J Clin Microbiol Infect Dis 22: 768–769. ARONOV, A. 2002. Comparative study of the ecology of three groupers (Epinephelinae, Serranidae) at the shallow rocky habitats of the Israeli Mediterranean coast. MSc Thesis, Tel-Aviv University, Israel.
ARONSON, J.D. 1926. Spontaneous tuberculosis in salt water fish. J Infect Dis 39: 315-320.
ASHBURNER, L. D. 1977. Mycobacteriosis in hatchery-confined Chinook salmon (Oncorhynchus tshawytscua Walbaum) in Australia. J Fish Biol 10: 523–528.
AUSTIN, D., A.B. AUSTIN 2007. Bacterial Fish Pathogens: Diseases of Farmed and Wild Fish, 4th ed. Praxis Publ. Ltd., Chichester, UK. BANCROFT, G.D., S A. TEVENS 1996. Theory and Practice of Histological Techniques. Fourth edition.Churchill Livingstone. New York.
BANNEROT, S.P.1984. The dynamics of exploited groupers (Serranidae): an investigation of the protogynous hermaphroditic reproductive strategy. Ph.D. Dissertation University of Miami, Coral Gables, Fla. 393 pp.
BELAS, R., FALOON, P., A. HANNAFORD 1995 . Potential applications of molecular biology to the study of fish mycobacteriosis. Annu Rev Fish Dis 5: 133–173.
BRUNO, D.W., GRIFFITHS, J., MITCHELL, C.G., WOOD, B.P., FLETCHER, Z.J., DROBNIEWSKI, F.A., AND HASTINGS, T.S.1998. Pathology attributed to Mycobacterium chelonae infection among farmed and laboratory-infected Atlantic salmon (Salmo salar). Dis Aquat Org 33: 101–109.
CHATTOPADHYAY, G.N. 1998. Chemical Analysis of Fish Pond Soil and Water. Daya Publishing House. New Delhi, India.
CHAUVET C. 981. Calcul par otolimétrie de la relation. Long. T - Age d’Epinephelus guaza (Linné 1758) de la Côte Nord de la Tunisie. Rapp Comm Int Mer Médit 27(5): 103-106.
CLARK, H. F., AND SHEPARD ,C.C.1963. Effect of environmental temperatures on infection with Mycobacterium marinum (balnei) of mice and a number of poikilothermic species. J Bacteriol 86:1057–1069.
COLORNI, A. 1992. A systemic mycobacteriosis in the European sea bass Dicentrarchus labrax cultured in Eilat (Red Sea). Isr J Aquacult Bamidgeh 44: 75–81.
CORNISH, A., AND HARMELIN-VIVIEN, M. 2004. Epinephelus marginatus. In: IUCN 2010. IUCN Red List of Threatened Species. Version 2010.1. . Downloaded on23 March 2010.
COUTANT, C.C.1998. What is normative for fish pathogens? A perspective on the controversy over interactions between wild and cultured fish. J Aquat Anim Health 10: 101-10.
DIAMANT, A., BANET, A., UCKO, M., COLORNI, A., KNIBB, W., AND KVITT, H.2000. Mycobacteriosis in wild rabbitfish Siganus rivulatus associated with cage farming in the Gulf of Eilat, Red Sea. Dis Aquat Org 39: 211–219.
ENZENSBERGER, R., HUNFELD, K.P., ELSHORST- SCHMIDT ,T., BOER, A., AND BRADE, V. 2002. Disseminated cutaneous Mycobacterium marinum infection in a patient with non-Hodgkin’s lymphoma. Infection 30: 393–395.
FAISAL, M., AND EISSA, A.E.2009. Diagnostic testing patterns of Renibacterium salmoninarum in spawning salmonid stocks in Michigan. J Wildl Dis 45 (2): 447–56.
FALKINHAM, J.O., NICHOLS, G., BARTRAM ,J., DUFOUR, A., AND PORTAELS, F.2004. Natural ecology and survival in water of mycobacteria of potential public health significance. In: Bartram J, Cotruvo JA, Dufour A, Rees G, Pedley S, editors. Pathogenic mycobacteria in water: a guide to public health consequences, monitoring and management. London: IWA Publishing; p. 15-25.
FENNESSY, S.T.1998. Biology and management of some sex-changing rockcods (Serranidae) from Southern Africa. in : Poissons et Pêches Africains Diversité et Utilisation, Coetzee L., Gon J., Kulongowski C.(eds), Grahamstown (South Africa) FISA/PARADI. 112 pp.
FRERICHS, G. N. 1993. Mycobacteriosis; nocardiosis. In: Inglis, V., R. J. Roberts, and N. R. Bromage (eds.). Bacterial Diseases of Fish. Halsted Press, New York, Pp. 219-233.
GAUTHIER, D.T., RHODES, M.W., VOGELBEIN, W.K. ,KATOR, H., AND OTTINGER ,C.A.2003. Experimental mycobacteriosis in striped bass Morone saxatilis. Dis Aquat Organ 54: 105-117.
GIAVENNI, R., AND FINAZZI, M. 1980. Tuberculosis in marine tropical fishes in an aquarium. J Wildl Dis 16: (2), 161-168.
GOWEN, R.J., AND BRADBURY, N.B.1987. The ecological impact of salmonid farming in coastal waters: a review. Oceanagr. Mar Biol Annu Rev 25: 563 -575. Guay, D.R.P. (1996) Nontuberculous mycobacterial infections. Ann Pharmacotherapy 30: 819-830.
HALL, P.O.J., HOLBY, O., KOLLBERG, S., AND SAMUELSON, M.O. 1991. Chemical flux and mass balances in a marine fish cage farm. 4. Nitrogen. Mar Ecol Prog Ser 89 : 81 - 91.
HALL, L. W. JR., ANDERSON, R.D., AND ALDEN, R.W. 2002. A ten year summary of concurrent ambient water column and sediment toxicity tests in the Chesapeake Bay watershed: 1990-1999. Environ Monit Assess 76: 311-352.
HARVELL, C.D., KIM, K., BURKHOLDER J.M., COLWELL ,R.R., EPSTEIN, P.R., AND GRIME, D.J.1999. Emerging marine diseases-climate links and anthropogenic factors. Science 285:1505.
HAWKE J.P. 2000. Bacterial disease agents. In: Encyclopedia of Aquaculture (ed. by R.R. Stickney), pp. 87-88. John Wiley & Sons, New York, NY.
HECKERT, R.A., ELANKUMARAN, S., MILANI, A., AND BAYA, A. 2001. Detection of a new Mycobacterium species in, wild striped bass in the Chesapeake Bay. J Clin Microbiol 39: 710-715.
HEDRICK, R. P., MCDOWELL, T., AND GROFF, J. 1987. Mycobacteriosis in cultured striped bass from California. J Wildl Dis 23: 391-395. Heemstra, P.C. ,and Randall, J.E. (1993) Groupers of the World. FAO Fisheries Synopsis No. 125, Vol. 16.
HOLBY, O., AND HALL, P.O.J. 1991. Chemical flux and mass balances in a marine fish cage farm. II. Phosphorus. Mar Ecol Prog Ser 70: 263 - 272.
HOLMES, G.F., HARRINGTON, S.M., ROMAGNOLI, M.J., AND MERZ, W.G. 1999. Recurrent disseminated Mycobacerium marinum infection caused by the same genotypically defined strain in an immunocompromised host. J Clin Microbiol 37: 3059-61.
INGLIS, V., ROBERTS, R.J., AND BROMAGE, N. R. 1993. Bacterial Diseases of Fish. London: Blackwell Scientific. Blackwell Science Publications, London, p 219-233.
ISENBERG, H.D. 1998. Essential Procedures for Clinical Microbiology. 1st Edn, Washington DC, American Society for Microbiology Press, pp: 187 - 197
JACOBS, J.M., STINE, C.B., BAYA, A.M., AND KENT, M.L. 2009. A review of mycobacteriosis in marine fish. J Fish Dis 32: 119-130.
JORY, D.E., AND IVERSON, E.S. 1989. Species profiles: life histories and environmental requirements of coastal fishes and invertebrates (South Florida) - black, red, and Nassau groupers. U.S. Fish and Wildlife Service Biological Report 82(11.110). U.S. Army Corps of Engineers, TR EL-82 - 4. 21 pp.
KANE, A.S., STINE, C.B., HUNGERFORD, L., MATSCHE, M., DRISCOLL, C., AND BAYA, A.M. 2007. Mycobacteria as environmental portent in Chesapeake Bay fish species. Emerg Infect Dis 13: 329-331.
KENT, P.T., KUBICA, G.P. (1985) Public health mycobacteriology: a guide for the level III laboratory. US Department of Healthand Human Services, Publication no. (CDC) 86 - 8230.Centers for Disease Control, Atlanta.
KNIBB, W., COLORNI, A., ANKAOUA, M., LINDELL, D., DIAMANT, A. , AND GORDIN, H.1993. Detection and identification of a pathogenic marine mycobacterium from the European seabass Dicentrarchus labrax using polymerase chain reaction and direct sequencing of 16 rDNA sequences. Mol Mar Biol Biotechnol 2: 225-232.
KULLAVANIJAYA, P. 1999. Atypical mycobacterial cutaneousinfection. Clin Dermatol 17: 153- 158.
LAHEY, T. 2003. Invasive Mycobacterium marinum infections. Emerg Infect Dis 9: 1496-1498.
MASTER, R.N. 1995. Mycobacteriology. In: Isenburg HD (ed) Clinical microbiology procedures handbook. Vol.1. American Society for Microbiology, Washington, DC, P 301-316.
MCVICAR, A. H. 1975. Infection of plaice, Pleuronectes platessa L., with Glugea (Nosema) stepl~ani (Hagenmijller, 1899) (Protozoa: Microsporidi in a fish farm and under experimental condirions. J Fish Biol 7: 611-61.
MUNRO, A.L.S., MCVICAR , A.H., AND JONES R.1983. The epidemiology of infectious disease in comnlercially important wild marine fish. Rapp PV Cons Int Explor Mer 182: 21-32.
NIGRELLI, R. F., AND VOGEL, H. 1963. Spontaneous tuberculosis in fishes and in other cold-blooded vertebrates with special reference to Mycobacterium fortuitum Cruz from fish and human lesions. Zoologica 48: 130-143. NOGA, E. J. 1995. Fish Diseases: Diagnosis and Treatment. Mosby, St. Louis, Missouri. Pp. 156-158.
NYKAW AND O NEILL, E.F. 1970. A new approach to the study of non-acid fast mycobacteria. Ann New York Acad Sci 174: 862-871.
PAUL, D., AND GULICK, P.1993. Mycobacterium marinum skin infections: two case reports. J Family Pract 36(3): 336-338.
PUTTINAOWARAT, S. 1999. Detection and characterisation of aquatic Mycobacterium Spp. PhD dissertation, University of Stirling, Stirling, UK.
RANGER, B. S., MAHROUS, E. A., MOSI ,L., ADUSUMILLI, S., LEE, R. E., COLORNI, A.,RHODES, M., AND SMALL, P.L.C. 2006. Globally distributed mycobacterial fish pathogens produce a novel plasmid-encoded toxic macrolide, mycolactone F. Infect Immun 74: 6037–6045.
RHODES, M.W., KATOR, H., KAATTARI, I., GAUTHIER, D., VOGELBEIN, W., AND OTTINGER, C.A. 2004. Isolation and characterization of mycobacteria from striped bass Morone saxatilis from the Chesapeake Bay. Dis Aquat Org 61: 41–51.
ROSS, A. J.1970. Mycobacteriosis among salmonid fishes. In A Symposium on Diseases of Fishes and Shellfishes, Special Publication No. 5. Edited by S. F. Snieszko. Washington, DC: American Fisheries Society.
TORTOLI, E. 2003. Impact of genotypic studies on mycobacterial taxonomy: the new mycobacteria of the 1990s. Clin Microbiol Rev 16: 319–354.
TREASURER, J.W., AND COX, D. 1991. The occurrence of Aeromonas salmonicida in wrasse (Labridae) and implications for Atlantic salmon farming. Bull Eur Assoc Fish Path 11: 208-210.
VAN DUIJN, C. 1981. Tuberculosis in fishes. J Small Anim Pract 22: 391– 401.
VOGELBEIN ,W., ZWERNER, D., KATOR ,H., RHODES, M., KOTOB, S.I., AND FAISAL, M.1998. Mycobacteriosis in the striped bass, Morone saxatilis, from Chesapeake Bay. In: Kane AS, Poynton SL (eds) Proc 3rd Symp Aquat Anim Health, Aug 30–Sep 3, 1998, Baltimore. APC Press, Baltimore, MD, p 109.
WOLF, J.C., AND SMITH, S.A.1999. Comparative severity of experimentally induced mycobacteriosis in striped bass Morone saxatilis and hybrid tilapia Oreochromis spp. Dis Aquat Organ 38:191-200.
WOLKE, R.E., AND STROUD ,R.K.1978. Piscine mycobacteriosis. In: Montali, R.J. (Ed.), Mycobacterial infections of zoo animals. Smithsonian Institution Press, New York, pp. 86-104.
WOOD, J.E., AND ORDAL, E.J.1958. Tuberculosis in Pacific salmon and steelhead trout. Fish. Comm. Oregon, Contribution, 25, 1-38.
YIP, M. J., PORTER ,J. L., FYFE, J. A. M., LAVENDER, C. J., PORTAELS, F., RHODES, M. W., KATOR, H., COLORNI, A., JENKIN, G. A., AND STINEAR ,T.2007. Evolution of Mycobacterium ulcerans and other mycolactone-producing mycobacteria from a common Mycobacterium marinum progenitor. J Bact 189: 2021–202.
This paper was presented at the 4th Scientific Congress of Egyptian Society of Animal Management 25-28 October, 2010. Engormix.com thanks the authors and the organizing committee for this contribution.



.jpg&w=3840&q=75)