Isolation, Identification and Biochemical Characterization of Vibrios Species from Shrimp Hatcheries near Pondicherry Coast, Tamilnadu, India
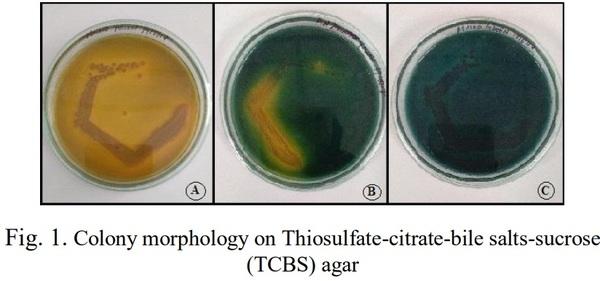
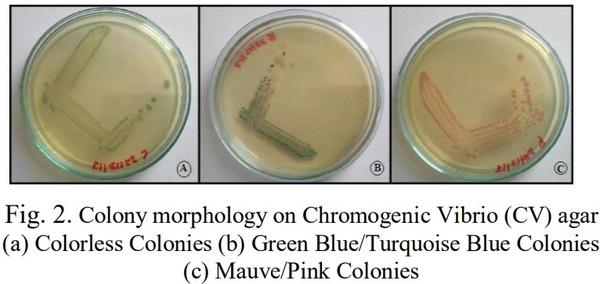
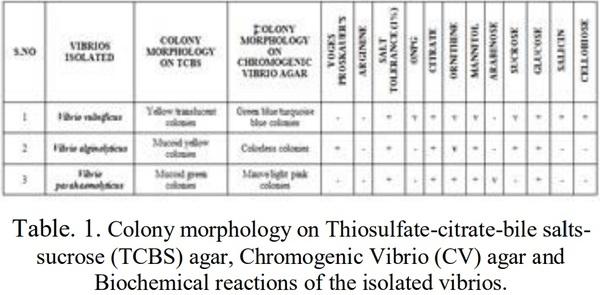
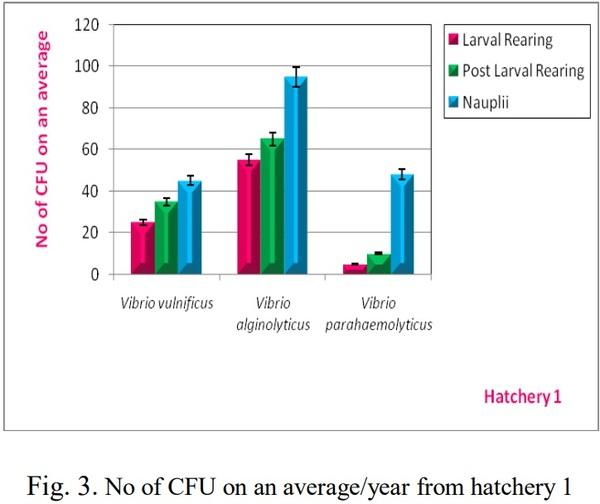
[1]. Couch, J. A. (1978). Diseases, Parasites and toxic responses of commercial penaeid shrimps of the Gulf of
Mexico and South Atlantic coasts of North American. Fishery
Bulletin, Vol. 76, (Pg. 1–44).
[2]. Overstreet, R. M. Marine maladies. (1978). Worms, germs and other symbionts from the Northern Gulf of Mexico.
Mississippi Alabama Sea Grant Consortium, Ocean Springs,
Mississippi, USA.
[3]. Lightner, D. V. (1983). Diseases of cultured penaeid prawn in J.P. McVeigh, editor. CRC Hand Book of
Mariculture, Vol. 1. Crustacean Aquaculture. CRC Press,
Boca, Raton, Florida, USA, (Pg. 289–320).
[4]. Lightner, D. V. (1985). A review of the diseases of cultured penaeid shrimps and prawns with emphasis on recent discoveries and developments in Proceedings of the first
International Conference. Culture of penaeid prawns and shrimps. Illio, Philippines, (Pg. 79-103).
[5]. Lightner, D. V. (1988). Vibrio disease of penaeid shrimp in C.J. Sindermann and D. V. Lightner, editors. Disease diagnosis and control in North American Marine Aquaculture,
2nd edition. Elsevier, New York, New York, USA, (Pg. 42-
47).
[6]. Lightner, D. V. (1196). Handbook of shrimp pathology and diseases of cultured penaeid shrimp. World Aquaculture
Society, Baton Rouge, Louisiana, USA.
[7]. Sindermann, C. J. (1990). Principal diseases of marine fish and shell fish, volume 2. Diseases of marine shell fish,
2nd edition. Academic Press, Inc.
[8]. Ruangpan, L., and Kitao J. (1991). Vibrio bacteria isolated from diseased tiger prawn, P. monodon. Journal of Fish
Diseases, Vol. 14, (Pg. 283–288).
[9]. Ruangpan, L., and Kitao J. (1992). Minimal inhibitory concentrations of 19 chemotherapeutants against Vibrio bacteria of shrimp Penaeus monodon. Pages 135–142 in M.
Shariff et al., editors. Diseases of Asian aquaculture. I. Fish
Health Section. Asian Fisheries Society, Manila, Philippines, (Pg. 135-142).
[10]. Chen, S. N., S. L. Huang, and G. H. Kou. (1992). Studies on the epizootiology and pathogenicity of bacterial infections in cultured giant tiger prawns, Penaeus monodon, in Taiwan.
Pages 195–205 in W. Fulkas and K. L. Main, editors. Diseases of cultured penaeid shrimp in Asia and the United States.
Hawaii Oceanic Institute.
[11]. Yang, J., X. Wu, and S. Zu. (1992). Observations on black spot on shell disease of cultivated penaeid shrimp by
SEM. Donghai Marine Science, Vol. 11, (Pg. 34–39).
[12]. De la Pena, L. D., T. Tamaki, K. Momoyama, Nakai, and
K. Muroga. (1993). Characteristics of the causative bacterium for vibriosis in the kuruma prawn, Penaeus japonocus.
Aquaculture, Vol. 115, (Pg. 1–12).
[13]. Jiravanichpaisal, P., T. Miyazaki, and C. Limsuwan. (1994). Histopathology, biochemistry and pathogenicity of
Vibrio harveyi infecting black tiger prawn, Penaeus monodon.
Journal of Aquatic Animal Health, Vol. 6, (Pg. 27–35).
[14]. Mohney, L., T. A. Bell, and D. V. Lightner. (1994). An epizootic of vibriosis in Equadorian pond-reared Penaeus vannamei Boone (Crustacea: Decapoda). Journal of World
Aquaculture Society, Vol. 25, (Pg. 116–125).
[15]. Lavilla-Pitogo, C. R. and L. D. de la Pena. (1998).
Bacterial diseases in shrimp (Penaeus monodon) culture in
Philippines. Fish Pathology, Vol. 33, (Pg. 405–411).
[16]. Lavilla-Pitago, C. R., E. M. Leano, and M. G. Paner. (1998). Mortalities of pond-cultured juvenile shrimp, Penaeus monodon, associated with dominance of luminescent Vibrios, in the rearing environment. Aquaculture, Vol. 164, (Pg. 337–
349).
[17]. Sizemore R. K, Davis J. W. (1985). Source of Vibrio spp. found in the hemolymph of the blue crab Callinectes sapidus. J Invertebr Pathol, Vol. 46, (Pg. 109–110).
[18]. Brock, J.A. and Lightner, D.V. (1990). Diseases of
Crustacea. In: O. Kinne (ed.) Diseases of Marine Animals Vol.
3, Biologische Anstalt Helgoland, Hamburg, (Pg. 245-424).
[19]. Owens, L. and Hall-Mendelin. (1989). Recent Advances in Australian shrimps (sic) diseases and pathology. Advances in Tropical Aquaculture, Tahiti, AQUACOP, IFREMER,
Actes de Colloque, Vol. 9, (Pg. 103-112).
[20]. Owens, L., Muir, P., Sutton, D. and Wingfield, M. (1992). The pathology of microbial diseases in tropical
Australian Crustacea. In: M. Shariff, R.P. Subasinghe and J.R.
Authur (eds.) Diseases in Asian Aquaculture 1. Fish Health
Section, Asian Fisheries Society, Manila, Philippines, (Pg.
165-172).
[21]. Lavilla-Pitogo, C.R., Baticados, C.L., Cruz-Lacierda,
E.R. and de la Pena, L. (1990). Occurrence of luminous bacteria disease of Penaeus monodon larvae in the Philippines.
Aquaculture, Vol. 91, (Pg. 1-13).
[22]. de la Peña, L.D., Kakai, T, Muroga, K. (1995).
Dynamics of Vibrio sp PJ in organs of orally infected kuruma shrimp, Penaeus japonicus. Fish. Pathol, Vol. 30, (Pg. 39-45).
[23]. Nash, G. Nithimathachoke, C., Tungmandi, C.,
Arkarjamorn, A., Prathanpipat, P. and Ruamthaveesub, P. (1992). Vibriosis and its control in pond-reared Penaeus monodon in Thailand. In: M. Shariff, R.P. Subasinghe and
J.R. Authur (eds.) Diseases in Asian Aquaculture 1. Fish
Health Section, Asian Fisheries Society, Manila, Philippines, (Pg. 143-155).
[24]. Lavilla-Pitogo, C.R., Baticados, C.L., Cruz-Lacierda,
E.R. and de la Pena, L. (1990). Occurrence of luminous bacteria disease of Penaeus monodon larvae in the Philippines.
Aquaculture, Vol. 91, (Pg. 1-13).
[25]. Karunasagar, I., R. Pai, G. R. Malathi, and I.
Karunasagar. (1994). Mass mortalities of Penaeus monodon larvae due to antibiotic resistant Vibrio harveyi infection.
Aquaculture, Vol. 128, (Pg. 203–209).
[26]. Hameed, A. S. S., J. J. Farmer, F. W. HickmannBrenner, and G. R. Farming. (1996). Characteristics and pathogenicity of a Vibrio campbelli like bacterium affecting hatchery reared P. indicus (Milne Edwards 1837) larvae.
Aquaculture Research, Vol. 27, (Pg. 853–863).
[27]. Shome, R., B. R. Shome, and R. Soundararajan. (1999).
Studies on luminous Vibrio harveyi isolated from Penaeus monodon larvae reared in hatcheries in Andamans. Indian
Journal of Fisheries, Vol. 46, (Pg. 141– 147).
[28]. Hua Ji, Yan Chen, Yunchang Guo, Xiumei Liu, Jian
Wen, and Hong Liu. (2011). Occurrence and characteristics of vibrio vulnificus in retail marine shrimp in china. Food
Control, Vol. 22, Issue. 12, (Pg. 1935-1940).
[29]. do Nascimento S.M, dos Fernandes Vieira
R.H, Theophilo G.N, Dos Prazeres Rodrigues D, Vieira G.H. (2001). Vibrio vulnificus as a health hazard for shrimp consumers, Vol. 43, Issue 5, (Pg. 263-266).
[30]. Liu, C.H., Cheng W, Hsu J.P and Chen J.C. (2004).
Vibrio alginolyticus infection in the white shrimp Litopenaeus vannamei confirmed by polymerase chain reaction and 16S rDNA sequencing. Dis Aquat Organ, Vol. 61, Issue 1-2, (Pg.
169-174).
[31]. Vengadesh, L., Kok-Gan C and Learn-Han L. (2014).
Vibrio parahaemolyticus: a review on the pathogenesis, prevalence, and advance molecular identification techniques.
Front Microbiol, Vol. 5, (Pg. 705).
[32]. Patricia L.L., Antonio L.G, Ruth E.M, María del Carmen
F.M, Jesús A. Fierro-Coronado Píndaro Álvarez-Ruiz and
Genaro Diarte-Plata. (2016). Isolation and characterization of infectious Vibrio parahaemolyticus, the causative agent of
AHPND, from the whiteleg shrimp (Litopenaeus vannamei).
Lat. Am. J. Aquat. Res, Vol. 44, Issue 3, (Pg. 470-479).
[33]. Barman, D., Nen P, Mandal S.C, and Kumar V. (2013).
Immunostimulants for Aquaculture Health Management. J
Marine Sci Res Dev, Vol. 3, (Pg. 134).
[34]. Laurent, V., Geert R, Patrick S and Willy V. (2000).
Probiotic Bacteria as Biological Control Agents in
Aquaculture. Microbiol Mol Biol Rev, Vol. 64, Issue 4, (Pg.
655–671).
[35]. Nakayama, T., N. Nomura, and M. Matsumura. (2007).
The effect of copper concentration on the virulence of pathogenic Vibrio harveyi, Journal of Applied Microbiology,
Vol. 102, Issue 5, (Pg. 1300–1306).





.jpg&w=3840&q=75)