Immunological factors in Black Tiger shrimp
Immunological factors in Black Tiger shrimp, Penaeus monodon, Fabricius
The farming of marine shrimp is practiced worldwide and is estimated to provide over 25% of total shrimp production. In Asia, shrimp production has been predominantly focused on the Black Tiger prawn, Penaeus monodon. Successful shrimp production requires the use of effective disease prevention strategies and a good understanding of the basic immune functions of shrimp is important to understand how these can be implemented effectively. Both the cellular and humoral immune systems of the shrimp function synergistically to protect the shrimp and eliminate foreign particles and pathogens. Low immunity may be caused by factors such as poor water quality, disease and presence of toxins.
However, several factors, including trace nutrients (astaxanthin, vitamins and minerals), probiotics and immune stimulants (ß-glucan, peptidoglycan and lipopolysaccharide) have been reported to promote shrimp immunity and thereby increase resistance to disease. Genetics is also an important factor controlling both the immune system and defense mechanisms, and efforts to improve immunity and disease resistance of Black Tiger shrimp through genetic selection are being undertaken by researchers at different institutes in Thailand. This paper reviews Thai studies on the immune system and factors affecting the immune response in the Black Tiger prawn, Penaeus monodon.
Penaeus monodon is a crustacean that molts by shedding the exoskeleton periodically throughout the growth period. Molting is a critical process and the shrimp are vulnerable during the period when the newly formed exoskeleton is still undergoing hardening. At this time they are more prone to predation and infection by water-borne pathogens. In order to survive through several molting cycles, shrimp have evolved very efficient cellular and humoral immune systems that work together to protect the shrimp by eliminating foreign particles and invading pathogens. The exoskeleton is the primary barrier of the shrimp defense mechanism and, in healthy shrimp, it is translucent and smooth due to a coating of anti-fouling mucus produced by the tegumental gland underneath the subcuticular epithelium. This coating prevents the colonization of the shell by pathogens. In the event that the exoskeletal barrier fails to prevent infection, the internal immune defenses are triggered as a response to infection.
Cellular immune system
The cellular immune mechanism is fundamental in animals, with active phagocytic cells ingesting and eliminating invading particles. In P. monodon, this system involves a number of different cell types including hemocytes and fixed phagocytes.
HEMOCYTES
P. monodon possesses three different types of blood cells. Hyaline cells are small, with few or no cytoplasmic granules. There are also two types of granular hemocytes - semi-granular hemocytes and large granular hemocytes. The larger granular hemocytes have a greater number of cytoplasmic granules (Figures 1 and 2). In healthy P. monodon, total hemocyte counts range from 104 to 105 cell/mm3 while those maintained in an unfavorable environment (e.g. low oxygen content), or with bacterial or viral infections, show a marked drop in hemocyte count.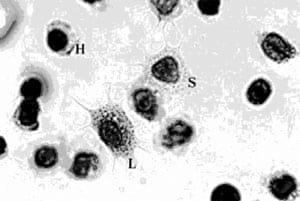
Figure 1.P. monodon blood cells, H = hyaline hemocyte, s = semi-granular hemocyte, L = large granular hemocyte (Geimsa's stain).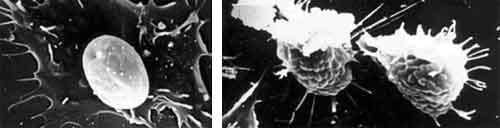
Figure 2.Characteristics of blood cell surfaces in healthy P. monodon (hyaline hemocyte on left, large granular hemocyte on right) viewed under the scanning electron microscope.
FIXED PHAGOCYTES
Fixed phagocytes are immobile phagocytes that engulf pathogens and foreign bodies. They are distributed in tissues such as gill, heart and various connective tissues (Figure 3). These cells are distinct from blood cells as they are immobile and do not possess granules but they act to augment blood cells in eliminating pathogens.
In general, both hemocytes and fixed phagocytes engulf foreign bodies by the process known as phagocytosis. In P. monodon, phagocytosis can be observed easily using light microscopy and is principally performed by the blood cells and fixed phagocytes. The formation of nodules and encapsulation of larger pathogens or foreign bodies by several layers of granular cells and fixed phagocytes are also found in various tissues such as the gill, lymphoid tissue and heart (Figure 4, Supamattaya et al., 2000a, c).
In addition to these physical processes, cellular secretions, which can be studied by determining the level or activity of associated proteins or the concentration of ions and free radicals, are also observed. In P. monodon, the cellular secretions that play a major role in self-defense are phenoloxidase enzyme and various intermediary metabolites.
PHENOLOXIDASE ENZYME
The granules in the granular hemocytes consist of precursors to prophenoloxidase. In the prophenoloxidase activating system, degranulation releases this enzyme from the granular hemocytes following invasion by foreign bodies or pathogens. This system is also triggered by detection of microbial compounds such as ß-glucan, peptidoglycan and lipopolysaccharides through specific binding proteins on the surface of the hemocytes. The prophenoloxidase 'cascade' process is responsible for producing and secreting toxic metabolites (e.g. quinone, which can be polymerized to melanin) that inhibit and eradicate the agents. The end product of this system appears as blackish nodules, usually around the lesions on the gill or exoskeleton (Soderhall and Cerenius, 1992).
Additionally, P. monodon with high levels of phenoloxidase activity in the blood are less susceptible to infection than those with lower levels of phenoloxidase. In normal P. monodon, enzyme levels range from 250-500 units/min/mg protein. When infection occurs, this increases following triggering of the system by invading microbes (or following the administration of immune stimulant compounds) then sharply drops if infection continues (Silakes and Supamattaya, 2000; Supamattaya et al., 2000d).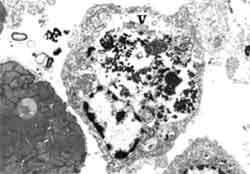
Figure 3. Electron microscopy reveals the characteristics of fixed phagocyte in P. monodon in heart tissue with white spot virus infection (V =vacuole with virus engulfed and digested).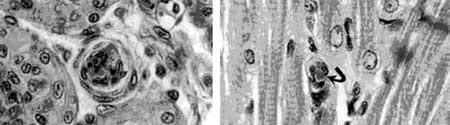
Figure 4. Entrapment of yeast by nodule formation in lymphoid organ of P. monodon (left) and by fixed phagocyte in heart tissue (right).
INTERMEDIATE METABOLITES
Reactive oxygen intermediates (ROIs) produced through respiration and other activities of blood cells are superoxide, hydrogen peroxide, hydroxyl radical and singlet oxygen. These metabolites are capable of eliminating invading agents particularly when the superoxide level increases as phagocytosis of microbes or foreign bodies takes place (Munoz et al., 2000). P. monodon that possess high levels of superoxide are more capable of resisting infection with luminescent bacteria (Vibrio harveyi) than those with lower levels. The level of superoxide in normal P. monodon ranged from 25- 30 units and correlated with phagocytic activity (Silakes and Supamattaya, 2000).
Apart from the protein and intermediate metabolites mentioned above, clotting protein in the blood cells of marine shrimp, including P. monodon, are responsible for the clotting of the hemolymph. This not only prevents loss of hemolymph, but also obstructs the entry of pathogens into the body. Pathogens trapped by the clot are then eliminated through other mechanisms. Under normal conditions, the blood of P. monodon clots rapidly on exposure to air or oxygen and clots much slower in shrimp with bacteria or viral infections. This results in a greater opportunity for subsequent invasion of pathogens into the body and it is common to find multiple infections in moribund or dead shrimp.
Humoral immune system
The humoral immune system in shrimp generally involves different biological compounds in the hemolymph that inhibit or eliminate foreign bodies and pathogens. In P. monodon, this system is composed of various substances.
LECTIN-BASED SYSTEMS
Lectin, or agglutinin, is a carbohydrate binding protein capable of binding several types of carbohydrate on the cell wall of pathogens or foreign bodies, causing the reaction known as agglutination. Following agglutination, the invading body is then eliminated through one or more of the other mechanisms described. Agglutinin functions in coordination with blood cells, which secrete some essential components that stimulate the agglutination reaction. In normal P. monodon, even hemolymph diluted 16 times retained a strong agglutination reaction (Sritanyalucksana, 1995)
MECHANISMS INVOLVING ANTIMICROBIAL COMPOUNDS
The production of antimicrobial compounds is another aspect of the humoral defense system that plays an important role in innate immunity. In shrimp, compounds that have been found to effectively inhibit and kill pathogens are bactericidin and other antimicrobial peptides.
Bactericidins are a class of compounds found in the hemolymph of several shrimp and crab species and are capable of inhibiting and eradicating bacteria. Some of these are specific to Gram(+) bacteria, while others are capable of eradicating both Gram(+) and Gram(-) bacteria as well as some parasites (Noga et al., 1996a). Bactericidins are also found in P. monodon and can efficiently inhibit bacterial proliferation, particularly when stimulated with killed bacterial cells (Sritanyalucksana, 1999).
Antimicrobial peptides are also capable of eradicating pathogens. The peptides are proteins of low molecular structure and inhibit and eradicate pathogens by increasing K+ inflow into the bacterial cells, causing the bacteria to lose control of their metabolism. Some peptides have structures that bond to the bacterial cell wall and inhibit cell proliferation. Penaeidins have been found in P. vannamei that inhibit bacteria and can also inhibit and eradicate Fusarium oxysporum, a mold that causes disease in shrimp (Destoumieux et al., 1999; Bachere et al., 2000). Another potential antimicrobial peptide is cecropin, produced from the larvae of silkworm, which also inhibits various types of bacteria. In the future, these peptides may be used as natural antimicrobials to control bacterial diseases. Apart from these systems that principally act
against bacteria and other pathogens, the search for antiviral compounds is of increasing interest due to the severity and impact of viral diseases in marine shrimp. P. japonicus survivors from Penaeid Acute Viremia (PAV) were found to contain a substance in the plasma that was believed to have some action as a 'neutralizing factor' since experiments showed that it could effectively eradicate the virus (Venegas et al., 2000). A similar result was reported in the case of Taura Syndrome Virus (TSV) in P. vannamei (Hasson et al., 1999). However, there is a lack of studies on the structure and biochemical characteristics of such substances due to limitations in the development of crustacean cell culture.
RESPONSES OF P. MONODON TO INFECTION AT GENE AND MOLECULAR LEVEL
There have been reports of spheroids in P. monodon that have survived infection with Yellow Head Virus (YHV) (Flegel et al., 1997), Mid-crop Mortality Syndrome Virus (MCSV) (Anggraeni and Owens, 2000) and in white shrimp that have survived TSV (Hasson et al., 1999). It is thought that the spheroids formed may be involved in virus resistance mechanisms in marine shrimp since the surviving shrimp are more resistant to these diseases. In addition, it was reported that White Spot Syndrome Virus (WSSV)-infected P. monodon have developed distinctive gene groups that differ from those in other shrimp species particularly when genes are detected on alignment with chromosome 21 (interferon/interleukin) (Graidist, 2001). In mammals this gene is responsible for the formation of cytokine, which inhibits the proliferation of virus and is involved in the process of inflammation (Cooper et al., 1996; Bondan, 2000). Further studies on the role of this gene and possible applications in shrimp health management are promising for shrimp culture.
This short introduction to the immune system in P. monodon and other marine shrimp species shows that they are capable of defending themselves from a range of diseases. It also allows us to understand the effect of external factors on the health of the shrimp and the proper functioning of the defense system and the importance of maintaining a balanced culture system to support a healthy immune system. Several factors can play a role in the immune system of shrimp and other crustaceans.
Immunological factors in P. monodon Culturing P. monodon to ensure rapid growth, good health, freedom from disease and a high survival rate requires knowledge and experience in shrimp farm operation. Monitoring the immune system can be an important means of indicating the health status of shrimp. Shrimp with low immunity are weak and more vulnerable to diseases, resulting in loss of productivity. To enhance the immune system of shrimp effectively requires an understanding of the factors affecting the immune system. Following are some of the major factors to be considered.
WATER QUALITY
The important water quality parameters in P. monodon grow-out ponds include physical parameters (such as transparency and temperature), chemical parameters (e.g. pH, dissolved oxygen (DO), hardness, salinity, lime requirement for soil, alkalinity, nitrite (NO2 -), nitrate (NO3 -), ammonia (NH3 +) levels) and biological factors (e.g. pond ecology, microbial species composition and abundance of plankton). These need to be maintained in balance to avoid stress on the shrimp.
Water quality has been shown to affect the immune system of P. monodon. Supamattaya et al. (2000e) found that dissolved oxygen, pH and temperature can affect the immune response of P. monodon (Figures 5-7). Low pH (<6), low DO or abrupt temperature change can reduce the capacity of blood cells to eliminate bacteria, and lower enzyme activity for elimination of foreign bodies. Thus, poor water quality can result in P. monodon becoming more susceptible to infection. The severity of subsequent infections depends on the types of pathogens present and the extent of the reduction in immune capacity caused by the suboptimal water quality.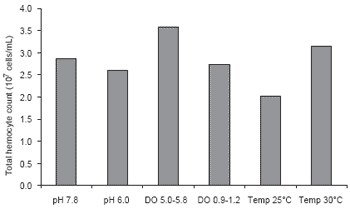
Figure 5. Effect of pH, dissolved oxygen (DO) and temperature on total hemocyte count in P. monodon.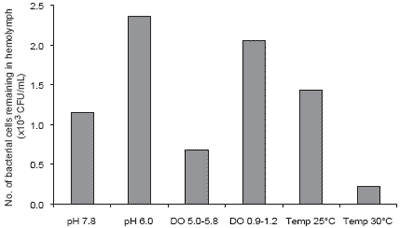
Figure 6.Effect of pH, dissolved oxygen (DO) and temperature on elimination of bacteria in plasma.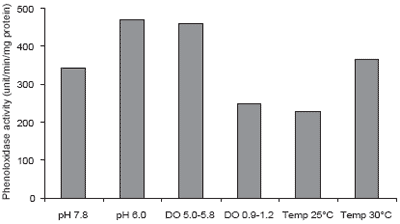
Figure 7.Effect of pH, dissolved oxygen (DO) and temperature on phenoloxidase activity.
This study showed that several environmental factors, particularly pH, DO, and rapid, wide temperature fluctuations all affect the immune system of P. monodon. When P. monodon are maintained in an unsuitable environment over a prolonged period, or come under stress as a result of environmental change or imbalance, the immune system is depressed and the shrimp become more susceptible to disease. Shrimp farmers should take care to ensure good control over important water quality criteria such as DO. Dissolved oxygen should be maintained above 4-5 mg/L throughout the day and night. A high DO supports good shrimp health, increased feed intake and growth as well as ensuring that other oxygen dependent pond processes are maintained. On the other hand, low DO levels, especially at night when phytoplankton photosynthesis stops, result in stress and greater vulnerability to disease.
Oxygen is not the only factor, however, as other water and bottom soil quality factors also have an impact on the shrimp immune system and productivity.
INFECTIOUS DISEASES
Disease problems are common in P. monodon culture. The most important and well-known diseases are caused by white spot syndrome virus, yellow head virus, and vibriosis, particularly the luminescent disease caused by Vibrio harveyi. The data in Figures 8-10 show the effect of these three diseases on shrimp immunity indicated by reductions in hemocyte count, enzyme and phagocytic activities compared to healthy P. monodon as a control (Supamattaya et al., 2000f).
Viral infection caused a marked drop in the efficiency of the P. monodon immune system and caused changes in the structure and characteristics of hemocytes (Figure 11). Virus infection also resulted in reduced hemocyte count and enzyme activity (Supamattaya et al., 2000b). This loss of immune status results in poor shrimp health and a higher level of vulnerability to co-infections and increased mortality.
It is clear that infectious diseases have a great effect on the composition of the hemolymph and the function of the immune system in P. monodon as these parameters drop markedly when comparing infected and healthy shrimp. This can be seen clearly when comparing the copper concentration in the plasma. Copper is an important component of the respiratory pigment of shrimp, which is in the form of hemocyanin. When P. monodon is infected with bacteria or virus, the copper concentration of the hemolymph declines sharply as does the hemocyanin (Table 1). This results in a reduction in oxygen transfer efficiency and a consequent rapid weakening of the shrimp.
MYCOTOXINS
Apart from problems associated with poor water quality and infectious disease, nutrition is another factor affecting the immune system in P. monodon. Poor quality feed or feed contaminated with toxins can produce adverse effects on growth and the function of the immune system. For example, the use of high quality feed and proper storage of feed without exposure to excessive moisture can reduce problems associated with mycotoxins, especially in the tropical zone.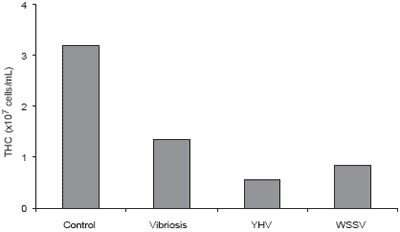
Figure 8.Effect of various diseases on total hemocyte count (THC) in P. monodon.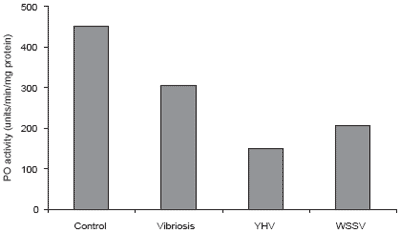
Figure 9.Effect of various diseases on phenoloxidase (PO) activity in P. monodon.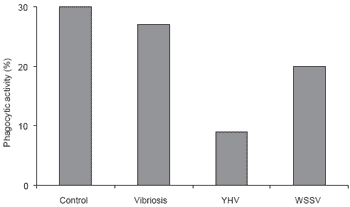
Figure 10.Effect of various diseases on phagocytic activity of blood cells of P. monodon.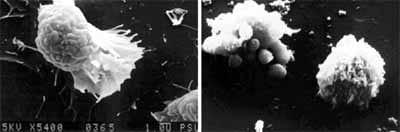
Figure 11.Characteristics of blood cells in healthy (left) and in yellow head virus infected (right) P. monodon observed under the scanning electron microscope.
Table 1. Copper concentrations in hemolymph of healthy and YHV-infected P. monodon1.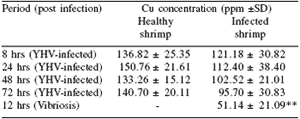
1Supamattaya et al. (1994).
*n = 30 **n = 10
Boonyaratpalin et al. (2000c) found aflatoxin B1 contamination at levels around 48 ppb in feed from 49 feed samples collected from farms across Thailand. Poor storage of feed on the farm was suspected since no toxin contamination was detected in 32 samples of freshly produced feed collected directly from feed producers. Proper feed storage on the farm is, therefore, very important to avoid mold and fungal growth and consequent mycotoxin contamination of the feed. Symptoms of mycotoxin contamination of shrimp are poor growth and, in severe cases, reddish body coloration in Black Tiger shrimp given feed contaminated with mycotoxins.
It was also reported that concentrations of mycotoxin above 74 ppb resulted in atrophy of hepatopancreatic tissue and diminution in the numbers of reserve cells (R-cell). In addition, if the shrimp were fed diets contaminated with mycotoxin at high levels, it resulted in severe changes in hepatopancreatic tissue such as degeneration of hepatic tubules with large quantities of hemocytes infiltrating the inter-tubular space. Toxins can also affect the hemolymph composition of shrimp, especially total hemocyte count, phenoloxidase activity, levels of alkaline phosphatase, SGOT and SGPT as shown in Table 2.
Table 2 shows the tendency towards increased hemocyte counts in P. monodon given feed containing 220 ppb alfatoxin B1. Slight differences were noted for cholesterol in the analysis of hemolymph composition and for the immune system while alkaline phosphatase differed greatly among treatments with a high variability within treatments. Both SGOT and SGPT tended to increase as aflatoxin B1 level increased. The shrimp immune system responded to the aflatoxin in a similar way as it reacts to infection or the presence of foreign bodies.
These studies showed that aflatoxin only affects P. monodon at relatively high concentrations and no residual aflatoxin was detected in shrimp muscle and head tissue after 8 weeks of feeding with contaminated feed. Neither the mold nor the mycotoxin were detected in the feed as supplied by the feed manufacturer or in the feed production process. This information is important in the light of concerns over the possibility of trade barriers being imposed through the potential for mycotoxin residues in exported shrimp meat.
PIGMENTS
Carotenoid pigments play an important role in aquaculture. This is well recognized in trout and salmon culture since these species require the addition of pigment in the feed in order to produce the characteristic red or orange-red color favored by consumers. For marine shrimp, there is a demand for quality shrimp of a suitable weight and size, free from residues or contamination by disease agents and with an attractive, dark red color after cooking.
P. monodon, like other marine shrimp, are incapable of synthesizing carotenoid pigments and these must be provided in the feed in the form of astaxanthin. Other precursors such as ß-carotene, zeaxanthin and canthaxanthin can be converted to astaxanthin and stored in the body. Supplementation of the feed with pigment can consist of synthetically produced forms of carotenoid such as astaxanthin, or ß-carotene. Natural sources of pigment can also be used. Some carotenoid-rich algal species such as the cyanophyte, Spirulina spp. or the microalga Dunaliella salina, can be used to provide pigment in the form of ß-carotene or zeaxanthin, which shrimp can convert into astaxanthin.
Carotenoids are also precursors for synthesis of vitamin A and are effective antioxidants. Studies of aquatic species, including shrimp and fish, show that astaxanthin not only induces darker color of P. monodon, but also enhances the function of the shrimp immune system and disease resistance. P. monodon, for example, developed higher resistance against bacteria (Table 3) when they were given astaxanthin-supplemented feed (Boonyaratpalin et al., 2000b).
Table 2. Effects of aflatoxin B1 on P. monodon hemolymph parameters.
To enlarge the image, click here
Table 3.Effects of astaxanthin on resistance against luminescent bacteria.
IMMUNE STIMULANTS
Immune stimulants are used to enhance the efficiency of the immune system in coping with pathogens. Microbial compounds such as ß-glucan from the yeast cell wall, and peptidoglycan and lipopolysaccharide from bacterial cell walls, are capable of stimulating the non-specific immune function of crustaceans and other animals (i.e. phagocytosis, degranulation of granular hemocytes, prophenoloxidase enzyme). Phenoloxidase plays an important role in stimulating phagocytosis and acceleration of clotting (Supamataya et al., 2000a).
ß-glucan
ß-glucan, a component of the cell wall of yeasts and fungi, enhances the shrimp immune system by stimulating the pro-PO system to eradicate invasive agents. Sittipun et al. (2000) extracted ß-glucan from Saccharomyces cerevisiae TISTR 5088 and added it to P. monodon feed. In shrimp fed a crude ß-glucan, enhanced immunity, higher hemocyte count, higher bacterial clearance rates and better resistance to bacteria were noted. P. monodon given feed with pure ß-glucan supplementation achieved the best results (Table 4).
Table 4.Total hemocyte count and bacterial clearance in P. monodon hemolymph after receiving dietary treatments over a 45 day period.
abMeans in a column with different superscripts differ (P<0.05)
Initial number of bacterial cells = 1.5 x 108 CFU/mL
*n=20
Peptidoglycan
Peptidoglycan (PG) is a component that strengthens the bacterial cell wall, particularly in Gram(+) bacteria, where PG constitutes 90% of the cell wall compared to only 5-20% in Gram(-) bacteria. Boonyaratpalin et al. (2000a) reported that supplementation of high and low molecular weight peptidoglycan (hm-PG and lm-PG) extracted from Bifidobacterium thermophilum added to P. monodon feed, resulted in high superoxide anion production by blood cells and a better capacity to clear bacteria from hemolymph (Table 5). Better resistance to Vibrio harveyi and higher survival of PG-treated shrimp were also noted (Table 6). With respect to superoxide anion production and bacterial resistance, lm-PG had a better effect than hm-PG. Similarly, supplementation of feed with peptidoglycan extracted from Brevibacterium lactofermentum enhanced survival rate, growth and stress tolerance (Itami et al., 1993).
Table 5.Effects of high and low molecular weight peptidoglycan (hm-PG and lm-PG) on superoxide anion, phenoloxidase activity and bacterial clearance in P. monodon given experimental feed over a 5 wk period.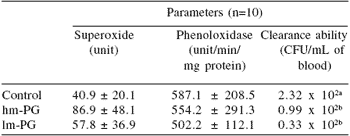
abMeans differ, P<0.05
Lipopolysaccharide (LPS)
LPS, present in Gram(-) bacteria such as Salmonella typhirium, Escherichia coli and Aeromonas salmonicida, can stimulate phagocytosis of blood cells of lobster and red snapper. Takahashi et al. (2000) studied the effect of LPS on resistance to Penaeid Acute Viraemia (PAV) in P. japonicus by supplementing it in the feed. They found that both phagocytosis and phenoloxidase activity were increased and resistance to PAV was also enhanced. Silakes and Supamattaya (2000) studied the production of vaccine from Vibrio harveyi by disrupting the cell wall and preparing it in powder form. The resulting product was included in the feed at 0.5-1.0% and fed to P. monodon. Results showed that LPS could effectively stimulate immunity in P. monodon. By contrast, the application of the LPS by immersion resulted in poor levels of immunity although repeated immersion over a short period could improve effectiveness.
Table 6.Effect of high and low molecular weight peptidoglycan (hm-PG and lm-PG) on P. monodon survival (%) after Vibrio harveyi infection.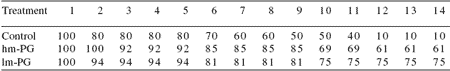
All of the immune stimulant studies conducted so far were conducted under laboratory conditions with the aim of developing methods for practical application. However, the effective application of these products depends on a number of factors such as dose, shrimp health, water and soil quality etc., and application under farm conditions should take these factors into consideration.
PROBIOTICS
The term 'probiotic' was first used by Parker in 1974 and was defined as 'microbes or substances that maintain the balance of gut microbes'. Later, Havenaar and Hius (1992) provided a definition of a probiotic as single or multiple types of microbes with beneficial effects on animals. The application of probiotic microbes is normally through supplementation of the feed. Adding probiotics to the feed can also augment the immune status of P. monodon, and can reduce or eliminate dependence on antibiotic treatments that can lead to antibiotic resistant strains of pathogens and residues in shrimp tissue. Appropriate probiotic microbes for aquatic species should be beneficial to host health, improve feed efficiency and nutritional value, stimulate host immunity, improve innate disease resistance of the host, improve the ambient environment, control proliferation of pathogenic microbes, facilitate digestion and, in some cases, improve water quality. This definition of probiotics excludes microbes that serve only as food for the host and which have no activity or influence on other microbes or the host environment.
The application of antibiotics to control infectious diseases in aquatic animals can result in the pathogen developing resistance and the presence of antibiotic residue in the flesh can adversely affect domestic and export markets and consumer perceptions of food safety of farmed seafood products. Several studies have attempted to develop new, non-antibiotic techniques to control pathogens. Application of probiotics is one solution to reduce or eliminate antibiotic use in aquaculture. Studies have shown that some strains of microbes can reduce the levels of pathogenic bacteria and have a beneficial effect on production in the aquatic environment (Moriarty, 1998).
In Thailand, probiotics must be registered at the Feed Quality Control Unit of the Department of Livestock Development, Ministry of Agriculture and Cooperatives. Various forms are available, ranging from products with single microbe species or strains (e.g. yeast strains or bacterial strains such as Streptococcus faecium and Bacillus subtilis) and those containing a mixture of two or more strains (e.g. mixtures of Lactobacillus acidophilus and S. faecium, or mixtures of yeast, Lactobacillus spp. and Streptococcus spp.). The commercial products can be in liquid or powder form with a carrier such as ground yellow corn, corn gluten, paraffin oil, peptone, dextrose, rice bran, calcium silicate, wheat bran, sucrose, lactose and others. The Animal Feed Control Act of 1982 requires that probiotics supplemented in animal feeds contain no less than 1 x 105 CFU/kg.
The application of probiotics in aquaculture has increased greatly in recent years and this trend looks set to continue. Research findings indicate that the correct application of probiotics can enhance productivity and prevent the occurrence of several diseases, as well as minimizing problems related to dependence on chemicals and antibiotics.
VITAMINS AND MINERALS
Vitamins, whether produced by the animal or obtained through the diet, are essential for many physiological processes. Most studies on the effects of vitamins on the immune system have been conducted in humans, terrestrial animals and fish but little is known of the effects of vitamin supplements on immune function of shrimp. Vitamin and mineral deficiencies are known to cause loss of production in terms of value and quality of cultured P. monodon.
Important vitamins for shrimp are vitamin C, choline, inositol, thiamin and pyridoxine. Vitamin C is essential and there have been many studies of its effect on physiology, growth and disease resistance. Pacific white shrimp (Litopenaeus vannamei) with vitamin C deficiency exhibited poor growth, poor feed conversion low molting frequency or even molting failure, and were less tolerant to stress. In severe cases, there was a failure in collagen synthesis in the exoskeleton which affected the healing process and resulted in dark lesions on the shell (Lightner et al., 1997), which he and Lawrence (1993) sometimes referred to as 'Black Death'.
Studies on vitamin C requirements of P. monodon showed that deficiency symptoms included abnormal gill cover development in which the gill cover appears opened and curled upwards. Other, less specific symptoms were poor growth, slow rate of healing, reduced tolerance to stress and reduced disease resistance. Although some crustaceans, such as the lobster Homarus americanus, are capable of synthesizing vitamin C, other species, including the penaeid shrimp P. japonicus (Kanazawa, 1994) and P. monodon, cannot and vitamin C supplementation of the feed is essential for shrimp growth.
Among the key macrominerals essential for growth of P. monodon are calcium and phosphorus. In general, P. monodon is capable of absorbing some minerals from the water while minerals can be lost at molting. P. aztecus is also reported to absorb calcium directly from the water but nutritional supplementation of phosphorus is necessary due to the low level of phosphorus in seawater (Kanazawa, 1994). Nutritional supplementation with high levels of calcium has no effect on growth and survival of P. aztecus but can stimulate metabolic processes for formation of the exoskeleton (Kanazawa, 1994).
GENETICS
Genetics is another factor governing the production characteristics of animals. In principle, animal breeding programs require good parent stocks with positive heritable traits such as rapid growth, resistance to disease and other important selection criteria. This requires careful planning of the breeding program and selection of appropriate broodstock to ensure the selection and development of breeding lines with characteristics that will benefit production.
It is well known that the quality and suitability of shrimp larvae and postlarvae depends a great deal on the quality of the broodstock used. However, after stocking in ponds, other factors that interact with the genetic factors to ensure good health and rapid growth are the environment and the feed. It is necessary that farmers should understand something about the interactions between these factors to achieve successful production.
In nature, P. monodon spend the major part of their life cycle in brackish water habitats. Larvae and juveniles grow in shallow water and migrate to deep water to breed and spawn. The estuarine areas in which the juveniles grow are subject to continuous change, either through natural or anthropogenic sources. Tidal regimes, sedimentation, freshwater influence and establishment of man-made infrastructure in the coastal area can all result in changes in the natural flora and fauna on which the shrimp feed and may also introduce toxic substances such as oil or pesticides into the environment. In the short term, these factors can affect the growth and survival of P. monodon and over the long term may result in genetic changes to P. monodon populations. Genetic changes in shrimp populations caused by such interactions have not been intensively studied. It is possible that adverse effects may include changes in the size and function of organs such as the ovary with reductions in fecundity or reproductive success, or disruption of the function of the digestive gland as has been shown in P. aztecus and P. setiferus (Castile and Lawrence, 1991). It is possible therefore, that shrimp growing in such unfavorable environments will be subject to undesirable genetic effects on the population that could in turn affect the production of cultured shrimp.
Recognizing such problems in natural shrimp stocks, researchers are now working to establish techniques for the production of high quality, domesticated broodstock to produce good quality larvae. Recent attempts have focused on development of specific pathogen free (SPF) founder stocks, selection of shrimp for desirable traits and identification of genes governing growth and immunity. In the case of P. monodon, application of cDNA techniques and subtractive hybridization are being used to identify the proteins related to the immune system by differentiating the mRNA between infected and uninfected shrimp. Graidist (2001) attempted to establish a cDNA library to detect specific products in the hemolymph of WSSV-infected shrimp. It is possible that putative protein syntenin (TE8), which acts as an adaptor adhering to PDZ-binding protein is involved in response or resistance to WSSV infection (Bangrak et al., 2002). This kind of information could be used in future to improve techniques for production of good quality P. monodon.
Conclusions
All organisms possess some degree of disease resistance. These depend on three main factors, i.e. genetics, environment and nutrition. In P. monodon, genetic factors are still largely uncontrollable due to the dependence on wild, natural broodstock rather than domesticated lines. Further research is required to enhance disease resistance in P. monodon through genetic improvement. In the case of wild stocks, it may be that establishment of good quality natural habitats such as artificial reefs may allow natural populations to recover genetic variability. This will require government policy to establish such zones and control the harvest of stocks.
Because of the important influence of the environment and feed on the immune system, shrimp farmers should maintain a good balance in the culture system to enhance shrimp health. Incorrect application of chemotherapeutics can adversely impact the immune system of shrimp, and should be avoided. Feeds should be nutritionally adequate and properly stored on the farm to avoid fungal and other contamination that may affect immune competence. Finally, the use of feed additives and supplements, vitamins, herbal therapeutics and probiotics may also allow the management of shrimp health and reduce or eliminate reliance on chemotherapeutics.
References
Anggraeni, M.S. and L. Owens. 2000. The haemocytic origin of lymphoid cells in the penaeid prawn Penaeus monodon. Dis. Aquat. Org. 40:85-92.
Bachere, E., D. Destoumieux and P. Bulet. 2000. Penaeidins, antimicrobial peptides of shrimp: a comparison with other effectors of innate immunity. Aquaculture 191:71-88.
Bangrak, P., P. Graidist, W. Chotigeat, K. Supamattaya and A. Phongdara. 2002. A syntenin-like protein with postsynaptic density protein (PDZ) domains produced by Black Tiger shrimp Penaeus monodon in response to white spot syndrome virus infection. Dis. Aqua. Organisms 49:19-25.
Boonyaratpalin, M., K. Supamattaya and C. Borisuth. 2000a. The immune system in Black Tiger shrimp, Penaeus monodon Fabricius: VIII. Effects of astaxanthin on blood parameters, immune system and disease resistance in Black Tiger shrimp, Penaeus monodon Fabricius. Songklanakarin J. Sci. Technol. 22(Suppl.):633-639 (in Thai).
Boonyaratpalin, M., K. Supamattaya, J. Pongmaneerat, S. Boonyaratpalin and Y. Toride. 2000b. Immunostimulant and vaccination in Black Tiger shrimp, Penaeus monodon Fabricius: IV. The stimulatory effect of high and low molecule peptidoglycan (PG) on immune responses in Black Tiger shrimp, Penaeus monodon Fabricius. Songklanakarin J. Sci. Technol. 22(Suppl.):689-696 (in Thai).
Boonyaratpalin, M., K. Supamattaya, D. Suprasert and C. Borisuth. 2000c. The immune system in Black Tiger shrimp, Penaeus monodon Fabricius: IX. Effects of aflatoxin B1 on growth performance, blood components, immune function and histopathological changes in Black Tiger shrimp, Penaeus monodon Fabricius. Songklanakarin J. Sci. Technol. 22(Suppl.):641-652 (in Thai).
Bondan, C. 2000. The function of type I interferons in antimicrobial immunity. Curr. Opin. Immunol. 12:419-424.
Castile, F.L. and A.L. Lawrence. 1991. Reproductive studies concerning natural shrimp populations: a description of changes in the size and biochemical composition of the gonads and digestive glands in Penaeid shrimp. In: Frontiers of Shrimp Research (W.J. Deloach, W.J. Dougherty and M.A. Davidson, eds). Elsevier Science Ltd., Amsterdam, pp. 17-32.
Cooper, E.L., M.H. Mansour and H.I. Negm. 1996. Marine invertebrate immunodefense responses: molecular and cellular approaches in tunicates. Ann. Rev. Fish Dis. 6:133-149.
Destoumieux, D., P. Bulet, J.M. Strub, A. van Dorsselaer and E. Bachere. 1999. Recombinant expression and range of activity of penaeidins, antimicrobial peptides from penaeid shrimp. Eur. J. Biochem. 266:335-346.
Flegel, T.W., S. Boonyaratpalin and B. Withyachumnarnkul. 1997. Current status of research on yellow head virus and white spot virus in Thailand. In: Diseases in Asian Aquaculture III (T.W. Flegel and I.H. MacRae, eds). Asian Fisheries Society, pp. 285-296.
Graidist, P. 2001. Genes involved in immune system of Black Tiger prawn (Penaeus monodon). MS Thesis, Prince of Songkla University, Thailand, p. 114 (in Thai).
Hasson, K.W., D.V. Lightner, L.L. Mohney, R.M. Redman and B.M. White. 1999. Role of lymphoid organ spheroids in chronic taura syndrome virus (TSV) infections in Penaeus vannamei. Dis. Aquat. Org. 38:93-105.
Havenaar, R. and J.H.J. Hius. 1992. Probiotics: a general view. In: The Lactic Acid Bacteria (B.J.B. Wood, ed). Vol. 1, Elsevier, London, pp. 151-169.
He, H. and A.L. Lawrence. 1993. Vitamin C requirements of the shrimp Penaeus vannamei. Aquaculture 114:305-306.
Itami, T., Y. Takahashi, E. Tsuchihira, H. Igusa and M. Kondo. 1993. Enhancement of disease resistance of Kuruma prawn, Penaeus japonicus, after oral administration of peptidoglycan. Proceedings of the Second Symposium on Diseases in Asian Aquaculture, 25-29 October 1993, Phuket, Thailand.
Kanazawa, A. 1994. The nutrition and feed of prawns and shrimp. In: Seminar on Aquaculture Feed and Disease (Takeda Vitamin and Food Asia PTE. Ltd.). Dusit Resort and Polo Club, Cha-Am, Hua Hin, Thailand, pp. 75-104.
Lightner, D.V., R.M. Redman, B.T. Poulos, L.M. Nunan, J.L. Mari and K.W. Hasson. 1997. Risk of penaeid shrimp viruses in the Americas by international movement of live and frozen shrimp. Rev. Sci. Technol. 16:146-160.
Moriarty, D.J.M. 1998. Control of luminous Vibrio species in penaeid aquaculture ponds. Aqaculture 164:351-358.
Munoz, M., Cedeno, R., Rodriguez, J., Knaap, W. P. W., Mialhe, E. and Bachere, E. 2000. Measurement of reactive oxygen intermediate production in haemocytes of penaeid shrimp, Penaeus vannamei. Aquaculture 191:89-107.
Noga, E.J., T.A. Arroll, R.A. Bullis and L. Khoo. 1996a. Antibacterial activity in haemolymph of white shrimp, Penaeus setiferus. J. Mar. Biotechnol. 4:181-184.
Silakes, S. and K. Supamattaya. 2000. Immunostimulant and vaccination in Black Tiger shrimp, Penaeus monodon Fabricius: II Production of vaccine from Vibrio harveyi and its application in Black Tiger shrimp, Penaeus monodon Fabricius. Songklanakarin J. Sci. Technol. 22(Suppl.):663-676 (in Thai).
Sittipun, M., A. Hanpongkittikul and K. Supamattaya. 2000. Immunostimulant and vaccination in Black Tiger shrimp, Penaeus monodon Fabricius: I. Extraction of beta-glucan from yeast and its application in Black Tiger shrimp, Penaeus monodon Fabricius. Songklanakarin J. Sci. Technol. 22(Suppl.):653-662 (in Thai).
Soderhall, K. and L. Cerenius. 1992. Crustacean immunity. Ann. Rev. Fish Dis. 2:3-23.
Sritanyalucksana, K. 1995. Study of the humoral defense factors in the Black Tiger prawn, Penaeus monodon. A thesis submitted in partial fulfillment of the requirements for the degree of master of science (Biotechnology), Faculty of Graduate Studies, Mahidol University, Bangkok, Thailand, p. 148.
Sritanyalucksana, K., P. Sithisan, B. Withayachumnarnkul and T.W. Flegel. 1999. A preliminary study on activation of agglutinin and antibacterial activity in hemolymph of the Black Tiger shrimp, Penaeus monodon. Fish Shellfish Immunol. 9:21-30.
Supamattaya, K., U. Ekpanithanpong, T. Itami and J. Kasornchandra. 2000a. The immune system in Black Tiger shrimp, Penaeus monodon Fabricius: I. Techniques on immunological assessment and blood component in Black Tiger shrimp, Penaeus monodon Fabricius. Songklanakarin J. Sci. Technol. 22(Suppl.):567-580 (in Thai).
Supamattaya, K., J. Kasornchadra and S. Boonyaratpalin. 1994. Comparative study of a simple method for the diagnosis of yellow-head baculovirus in the Black Tiger shrimp (Penaeus monodon). Songklanakarin J. Sci. Technol. 16:37-48 (in Thai).
Supamattaya, K., S. Kiattaptew and R.W. Hoffmann. 2000b. The immune system in Black Tiger shrimp, Penaeus monodon Fabricius: III. Electron microscopic studies on hemocytes of Black Tiger shrimp, Penaeus monodon Fabricius. Songklanakarin J. Sci. Technol. 22(Suppl.):589-596 (in Thai).
Supamattaya, K., W. Phromkunthong, C. Tantikitti and R. Hoffmann. 2000c. The immune system in Black Tiger shrimp, Penaeus monodon Fabricius: II Cells and tissues involved the removal of foreign particles in Black Tiger shrimp, Penaeus monodon Fabricius. Songklanakarin J. Sci. Technol. 22(Suppl.):581-588 (in Thai).
Supamattaya, K., J. Ruangsri, S. Kiriratnikom and N. Songsrichan. 2000d. The immune system in Black Tiger shrimp, Penaeus monodon Fabricius: IV Normal immuno-physiological values in Black Tiger shrimp, Penaeus monodon Fabricius. Songklanakarin J. Sci. Technol. 22(Suppl.):597-604 (in Thai).
Supamattaya, K., J. Ruangsri, S. Kiriratnikom and N. Suanyuk. 2000e. The immune system in Black Tiger shrimp, Penaeus monodon Fabricius: V. Effect of water temperature, DO and pH on immunophysiological functions in Black Tiger shrimp, Penaeus monodon Fabricius. Songklanakarin J. Sci. Technol. 22(Suppl.):605-614 (in Thai).
Supamattaya, K., J. Ruangsri, S. Kiriratnikom and N. Suanyuk. 2000f. The immune system in Black Tiger shrimp, Penaeus monodon Fabricius: VI. Effects of diseases on immuno-physiological functions and blood components in Black Tiger shrimp, Penaeus monodon Fabricius. Songklanakarin J. Sci. Technol. 22(Suppl.):615-621 (in Thai).
Takahashi, Y., M. Kondo, T. Itami, T. Honda, H. Inagawa, T. Nishizawa, G. Soma and Y. Yokomizo. 2000. Enhancement of disease resistance against penaeid acute viraemia and induction of virusinactivating activity in haemolymph of kuruma shrimp, Penaeus japonicus by oral administration of Pantoea agglomerans lipopolysaccharide (LPS). Fish Shellfish Immunol. 10:555-558.
Venegas, C.A., L. Nonaka, K. Mushiake, T. Nishisawa and K. Muroga. 2000. Quasi-immune response of Penaeus japonicus to penaeid rod shaped DNA virus (PRDV). Dis. Aquat. Org. 42:83-89.
Authors: K. SUPAMATTAYA1, V. CHITTIWAN1 and M. BOONYARATPALIN2
1 Aquatic Animal Health Research Center, Department of Aquatic Science, Faculty of Natural Resources, Prince of Songkla University, HadYai, Songkhla, Thailand
2 Department of Fisheries, Kasetklang, Bangkhen, Jatujak, Bangkok, Thailand


.jpg&w=3840&q=75)