Abstract: The present study was carried out to evaluate the effects of dietary supplementation of a commercial probiotic "Biogen®" on growth performance, carcass composition, blood hematological and biochemical parameters, histometric examination of fish dorsal muscles and economic efficiency of mono-sex Nile tilapia Oreochromis niloticus under different stocking densities. Therefore, fish with similar body weight (12.71 ± 0.17) were distributed randomly into seven treatments at different stocking densities, being 10 fish/ m3 which fed a basal diet without Biogen® (T1), 10 fish/m3 (T2), 20 fish/m3 (T3), 30 fish/m3 (T4), 40 fish/m3 (T5), 50 fish/m3 (T6) and 60 fish/m3 (T7), which were fed the basal diet but supplemented with 3g Biogen® Kg-1 diet for 14 weeks. The obtained results indicated that T4 was the best treatment which realized significantly (P ≤ 0.05) increases of all growth performance (final weight, AWG, ADG and SGR), hematological parameters (hemoglobin, RBCs count, PCV, blood platelets and WBCs count), plasma proteins (total protein, albumin, globulin and albumin/globulin ratio), improved FCR, blood indices (MCV, MCH and MCHC), differentiation of leukocytes, carcass composition, histometric examination of fish dorsal muscles and best economic efficiency. There were no adverse effects on water quality criteria among all experimental treatments. Consequently, from the obtained results, it could be concluded that the inclusion of the commercial probiotic Biogen® at a level of 3g Kg-1 diet at stocking density rate of 30 fish/m3 of mono-sex Nile tilapia O. niloticus is useful to get the best fish performance with friendly effects on the environment.
Key words: Nile tilapia- Biogen®- Probiotic- Stocking density- Fish physiology
INTRODUCTION
Tilapia species are used in commercial farming systems in almost 100 countries and are developed to be the most important fish for aquaculture in this century (Fitzsimmons, 2000). Nile tilapia, O. niloticus is currently considered to be the most important and commonly cultured tilapia species around the world, and constitutes over 70% of the cultured tilapia (Fitzsimmons, 2000 and 2004). In Egypt, tilapia production surpassed the production of common carp and thus tilapia has become the preeminent cultured fish species. Tilapias are often cultured in freshwater ponds without supplemental feeding. Intensification of culture practices necessitates the use of external feed input (Essa and Salama, 1994). Tilapia production in developing countries occurs primarily in semi-intensive ponds with fertilization and/or supplementary feeding (Tacon, 1990). Supplemental feeds are applied to increase fish yields above those produced with fertilization alone (Knud-Hansen and Batterson, 1994). In fish farming practices, stocking density is considered to be one of the important factors that affects fish growth, feed utilization and fish yield (Liu and Chang, 1992). Furthermore, Ellis et al. (2002)reported that stocking density (SD) is a key factor in determining the productivity and profitability of commercial fish farms. In tilapia, experiments on the effect of stocking density have been conducted on different fish sizes including fry and juveniles (El-Sayed 2002; Khattab et al., 2004b and Abdelhamid et al., 2007), sub-adults (Yi et al., 2004 and Bakeer et al., 2007) and large tilapia (Diana et al., 2004 and Rakocy et al., 2004).
The use of probiotics as farm animal feed supplements dates back to the 1970s. They were originally incorporated into feed to increase the animal''''''''''''''''s growth and improve its health by increasing its resistance to disease (Fuller, 1992).Today, probiotics are quite commonplace in health promoting "functional foods" for humans, as well as therapeutic, prophylactic and growth supplements in animal production and human health (Abdelhamid et al., 2000; Mombelli and Gismondo, 2000; Ouwehand et al., 2002; Sullivan and Nord 2002 and Senok et al., 2005). Many studies on probiotics in aquaculture have been used in in vitro models of specific bacteria as antagonists of pathogens (Vine et al., 2004 &2006 and Wang et al., 2008b). Other studies have been focused on growth promotion of fish by probiotic supplements (Gatesoupe 2002; Lara-Flores et al., 2003; EL-Haroun et al., 2006; Eid and Mohamed 2008 and Marzouk et al., 2008b) as well as on physiological and immune responses of fish by probiotic supplements (Khattab et al., 2004a; EL-Gohary et al., 2005 and Marzouk et al., 2008a).
Commercial probiotic "Biogen®" consists of Bacillus licheniformis and Bacillus subtilis. The advantage of these spore-forming bacteria is that they are able to survive the pelletization process. Biogen® can enhance the metabolism and energy of fish body cells, raise the efficiency of feed utilization and balance the secretion of various secretory glands. Moreover, it increases the vitality of cells by supplying oxygen to whole body, improves the immune responses, helps to excrete heavy metals, inhibits aflatoxin and maintains the normal endocrine system.
Biogen® has bactericidal effects and increases the palatability of feed, promotes the secretion of digestive fluids and stimulates the appetite (Mehrim, 2001). Moreover, Wang et al. (2008a) reported that with increasing demand for environment friendly aquaculture, the use of probiotics in aquaculture is now widely accepted. Therefore, the present study aimed to determine the effects of dietary supplementation of commercial probiotic "Biogen®" on growth performance, carcass composition, blood hematological and biochemical parameters, histometric examination of fish dorsal muscles and economic efficiency of mono-sex Nile tilapia O. niloticus under different stocking densities for 14 weeks.
MATERIALS AND METHODS
This study was conducted during the summer season 2007 in Fish Research Unit, Faculty of Agriculture, Mansoura University, Al-Dakahlia Governorate, Egypt. Mono-sex Nile tilapia O. niloticus fingerlings were gifted from the private hatchery in Tolombat 7 at Al-Reiad belonging to Kafr El-Sheikh Governorate. Fish were stocked into a rearing tank for two weeks as an adaptation period, during which they were fed on a basal experimental diet. Thereafter, apparently-healthy fish with similar body weight (12.71 ± 0.17) were distributed randomly into seven treatments (as three replicates per treatment) with different stocking densities, being 10 fish/ m3 which fed the basal diet without Biogen® (T1), and 10 fish/m3 (T2), 20 fish/m3 (T3), 30 fish/m3 (T4), 40 fish/m3 (T5), 50 fish/m3 (T6) and 60 fish/m3 (T7), which were fed the basal diet but supplemented with 3g Biogen® Kg-1 diet. Each tank (1m3 in volume) was constructed with an upper irrigation open, an under drainage, an air stone connected with electric compressor. Dechlorinated tap water was used to change one third of the water in each aquarium every day. Some fish were kept frozen at the start of the experiment for chemical analysis.
A basal diet (89.89% dry matter, 30.13% crude protein, 4.42% ether extract, 53.54% carbohydrates, 11.91% ash, 431.71 Kcal/100g DM gross energy and 69.79 mg CP/Kcal GE, P/E ratio), which was calculated according (Macdonald et al.,1973) in form of; GE (Kcal/100 g DM) = CP x 5.64 + EE x 9.44 + Carbohydrates x 4.11. It was formulated from the commercial ingredients (fish meal 22%, soybean meal 27%, yellow corn 21%, wheat bran 20%, corn oil 3%, vit. & min. mixture 2% and molasses 5%). The dietary ingredients and Biogen® supplement were bought from the local market. Feed ingredients were ground and the different ingredients were mixed manually by warm water and molasses. The big part from the ingredients was milled and mixed well with adding 3g probiotic Biogen® Kg-1 diet according to Mehrim (2001) and the other part of feed ingredients (without Biogen®) also was milled and mixed well. The two parts of the diet were pressed by manufacturing machine (pellets size 1mm). During the experimental period (14 weeks), the fish were fed the experimental diets at a rate of 5% of the live body weight daily, six days a week during the first 7 weeks and 4% feeding rate of the live body weight at the other 7 weeks of the experiment. Experimental diets were introduced by hand twice daily, at 8 a.m. and 2 p.m. The amount of food was adjusted bi-weekly based on the actual body weight changes. Light was controlled by a timer to provide a 14h light: 10h dark as a daily photoperiod. Commercial probiotic Biogen® is a natural non-antibiotic feed supplement comprised of allicin (aged garlic extract) not less than 0.247 m mol./g, Bacillus subtilis nato (6 x 107 cells/g), high unit hydrolytic enzymes not less than 3690 units/g (amylolitic, lipolytic, prolytic and cell separating enzymes), germanium (ginseng 41.98 ppm of Ge. element) and organic selenium (Mehrim, 2001).
At the end of the experiment, the remained fish were sampled from each tank and kept frozen for chemical analysis. The chemical analyses of the basal diet and whole fish body were carried out according to the AOAC (2000). Water quality parameters were measured weekly (Abdelhamid, 1996) including temperature (via a thermometer), pH (using Jenway Ltd., Model 350-pH-meter) and dissolved oxygen (using Jenway Ltd., Model 970- dissolved oxygen meter). Body weight of individual fish was measured bi-weekly to point feed quantity and to calculate growth performance according toAbdelhamid (2000)in form of: Average weight gain (g/fish) AWG = average final weight (g) - average initial weight (g), Average daily gain, (g/fish/day) ADG = AWG (g)/experimental period (days), Specific growth rate (SGR, %/day) = [ln final weight - ln initial weight] x 100/experimental period (d), Feed conversion ratio (FCR) = feed intake (g)/live weight gain (g) and Survival rate (SR%) = end number of the alive fish/the beginning number of the fish x 100.
At the end of the experiment, blood samples were collected from the fish caudal peduncle of the different groups. Adequate amounts of whole blood in small plastic vials containing heparin were used for the determination of hemoglobin (Hb) by using commercial kits (Diamond Diagnostic, Egypt). Also, total erythrocytes count (RBCs) and total leukocytes count (WBCs) were measured on an Ao Bright -Line Haemocytometer model (Neubauer improved, Precicolor HBG, Germany). Other blood samples were collected and transferred for centrifugation at 3500 rpm for 15 min to obtain blood plasma for determination of total protein according to Gornall et al., (1949), albumin according to Weichsebum (1946), globulin by difference according to Doumasand Biggs (1972). As well as, at the end of the experiment some fishes from all treatments were sacrificed and fish dorsal muscles were sampled. Samples were fixed in 10% neutralized formalin solution to histometric examination according to Pearse (1968).The data collected were statistically analyzed using one-way ANOVA adapted by SAS (1997). Means were statistically compared for the significance (P ≤ 0.05) using Duncan (1955) multiple range test.
RESULTS AND DISCUSSION
1-Rearing water quality properties
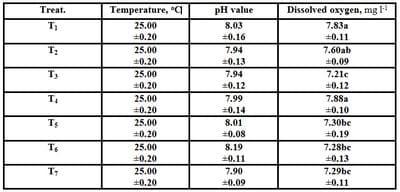
As shown from Table (1), all tested water quality criteria (temperature, oC; pH value; and dissolved oxygen, mg l-1) were suitable for rearing the experimental mono-sex Nile tilapia O. niloticus fingerlings. Although, there were no significant (P ≥ 0.05) differences in temperature oC and pH values by increasing the stocking densities of fish among all treatments; yet, dissolved oxygen (mg l-1) of T4 increased significantly (P ≤ 0.05) compared with other treatments, except T1. While, by increasing the stocking densities of fish the dissolved oxygen was decreased significantly (P ≤ 0.05), nevertheless this significant decrease was in the acceptable limits for rearing mono-sex O. niloticus fingerlings. These positive findings in water quality criteria related with good growth performance since there were no mortalities among all treatments (Table 2). These results are in agreement with those ofGall and Bakar (1999)they reported that dissolved oxygen (DO) decreased with increasing density of tilapia, being 6.7 mg l-1 at 20 fish/ l to 3.2 mg l-1 at 200 fish/l. On the other side, Abdelhamid et al. (2007) and Bakeer et al. (2007) reported that there were no significant differences in all water quality criteria measured by increasing the stocking densities of O. niloticus. Also, in the present study water quality criteria were suitable for rearing mono-sex O. niloticus fingerlings as cited by Abdelhamid (2000) and Abd El-Hakim et al. (2002).Moreover, Abdelhamid et al. (2007)tested water quality criteria and reported similar values which were suitable for rearing Nile tilapia fish.
Table (1): Effect of different stocking densities and dietary supplementation of Biogen® on quality parameters of rearing water of mono-sex Nile tilapia during the experimental period (Means ± SE)
a-c: Means in the same column different letters are significantly different (P ≤ 0.05).
2- Growth performance
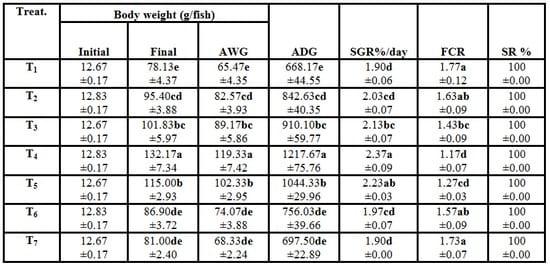
Results in Table (2) indicated that fish of T4 at 30 fish/m3 and 3g Biogen® kg -1 diet had significantly (P ≤ 0.05) increases in all growth performance parameters (final weight, average weight gain, average daily gain and specific growth rate) compared with the control group (T1, stocked at 10 fish/m3 without Biogen®). Also, the same fish group (T4) had significantly (P ≤ 0.05) improved feed conversion ratio compared with the other fish groups. However, by increasing the stocking density of fish (50 and 60 fish/m3 and fed diet supplemented with 3g Biogen®kg -1 diet, T6 and T7) there were no significant (P ≥ 0.05) differences in all above growth performance parameters compared with the control group (T1). Yet, there were no mortalities among all fish treatments. These positive effects in fish growth performance may be related with supplementation of commercial and natural probiotic Biogen®, which can enhance the metabolism and energy of fish body cells, raise the efficiency of feed utilization, increase the palatability of feed, promote the secretion of digestive fluids and stimulate the appetite (Mehrim, 2001). In this trend,Abdelhamid et al. (2007) reported that raising the stocking density (2, 3 and 4g fish liter -1) of the experimental Nile tilapia O. niloticus resulted in a significantly (P ≤ 0.05) decrease of the growth performance parameters (final weight, weight gain, average daily gain, relative growth rate and specific growth rate) of fish. However, they added that increasing the stocking density rate of fish led to significantly (P ≤ 0.05) increased feed conversion ratio of the experimental fish, but survival rate of fish was not influenced by raising the stocking density. Yet, they added that increasing dietary Betafin® (betaine) level caused a significant improve in this picture. As well as, Bakeer et al. (2007) found that body weight and length were negatively correlated to the stocking density of tilapia fish. Moreover, results of the present study are in agreement with those ofKhattab et al. (2004a) and Srour (2004)for tilapia and EL-Haroun (2007) for catfish. Also,EL-Haroun et al. (2006) reported that the growth performance and nutrient utilization of Nile tilapia were significantly (P ≤ 0.01) higher in the treatment receiving probiotic (Biogen®) than the control diet. Yet, Mohamed et al. (2007) reported that O. niloticus fingerlings fed on diets supplemented with probiotics exhibited greater growth than those fed the control diet. Also, they added that the diet containing 30% protein and supplemented with Biogen® at level of 0.1% produced the best growth performance. Recently, Eid and Mohamed (2008) revealed that using Biogen® at level of 0.1% was the best in terms of growth performance of mono-sex O. niloticus fingerlings. Moreover, Wang et al. (2008b) mentioned that tilapia (O. niloticus) supplemented with the probiotic bacterium, Enterococcus faecium ZJ4 showed significantly (P < 0.05) better final weight and daily weight gain (DWG) than those fed the basal diet (control). Recently,Marzouk et al. (2008b) reported that both fish groups fed on diet supplemented with probiotics (dead Saccharomyces cerevisae yeast and both of live Bacillus subtilis and Saccharomyces cerevisae) revealed significant (P < 0.05) increases in the body weight gain, specific growth rate and condition factor (K). While, they added that a significant (P < 0.05) decreased in feed conversion ratio was recorded comparison with the control group fed on probiotic-free diet. On the other hand, Abdelhamid et al. (2002) found that Biogen® supplementation (2 and 4 g kg -1 diet) did not significantly improve fish growth performance. Also, Diab et al. (2002)reported that Biogen® addition to fish diet at 0.5, 1.0 and 1.5% gave insignificant increase in fish growth performance.
Table (2): Effect of different stocking densities and dietary supplementation of Biogen® on growth performance of mono-sex Nile tilapia (Means ± SE)
a-e: Means in the same column different letters are significantly different (P ≤ 0.05).
AWG = Average weight gain (g/fish) ADG = Average daily gain (mg/fish/day)
SGR = Specific growth rate (%/d) FCR= feed conversion ratio.
SR = Survival rate (%).
3- Carcass composition of the experimental fish
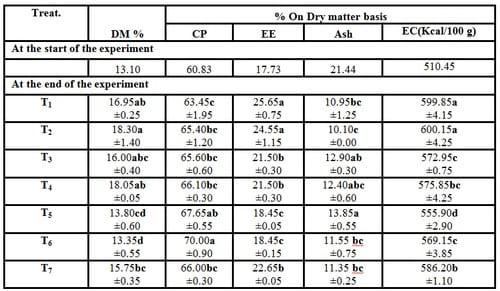
There were no significant (P ≥ 0.05) differences in dry matter, crude protein and ash of O. niloticus between T4 and T1. While, there were significant (P ≤ 0.05) decreased in both of ether extract and energy content in T4 compared with the control group (T1). However, by increasing the stocking densities of fish in treatments no significant (P ≥ 0.05) differences were recorded in crude protein and ash comparing with T1. While, increasing the stocking densities of fish led to significant (P ≤ 0.05) decreased in both of ether extract and energy content compared with the control group (Table 3). These positive effects in carcass composition of experimental fish may be due to the dietary supplementation with Biogen® which caused the good growth performance compared with the control group (Table 2). Since, Biogen® can enhance the metabolism and energy of fish body cells and raise the efficiency of feeds (Mehrim, 2001).
In this topic,Khattab et al. (2004b) reported that crude protein, total lipids and ash were significantly (P < 0.01) affected by protein level and increasing stocking density rate of tilapia fish. Yet, Abdelhamid et al. (2007) added that increasing the stocking density rate of fish was responsible for increased % of DM, leading to increases in CP and ash, but EE percentages of the whole fish body decreased. Yet, they added that increasing dietary Betafin® (betaine) level caused a significant improve in this picture. On the other side, results in the present study are in close agreement with those of Khattab et al. (2004a), Srour (2004), EL-Haroun et al. (2006) and Mohamed et al. (2007) for tilapia and EL-Haroun, (2007) for catfish. Moreover, Eid and Mohamed (2008)found that no statistical differences were observed in whole body moisture, crude protein, ether extract and ash of mono-sex O. niloticus fingerlings fed diets containing different levels of commercial feed additives (Biogen® and Pronifer®), compared with the control treatment.
Table (3): Effect of different stocking densities and dietary supplementation of Biogen® on carcass composition of mono-sex Nile tilapia (Means ± SE)
a-d: Means in the same column different letters are significantly different (P ≤ 0.05).
DM = Dry matter (%) CP = Crude protein (%)
EE = Ether extract (%) EC= Energy content (Kcal/100g), calculated according to Macdonald et al. (1973).
4-Blood hematological and biochemical parameters
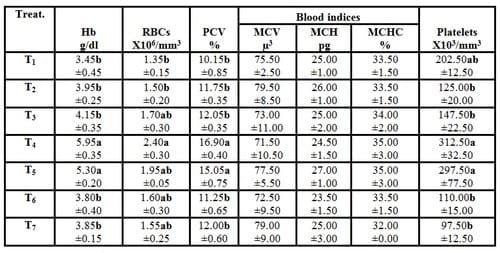
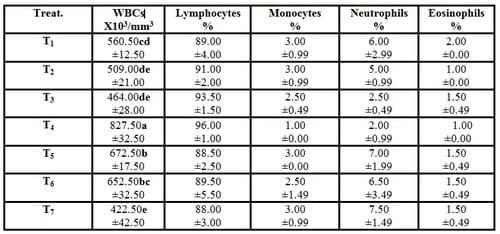
Results in Tables (4 and 5) showed that hemoglobin concentration, RBCs count, PCV%, blood platelets (thrombocytes) count and WBCs count in the experimental fish of T4 were increased significantly (P ≤ 0.05) compared with the control. While, no significant (P ≥ 0.05) differences were recorded in blood indices (MCV, MCH and MCHC) and differentiation of leukocytes among all experimental treatments. On the other side, by increasing the stocking densities of fish, no significant (P ≥ 0.05) differences were recorded in all above mentioned blood parameters compared with the control (T1). However, T7 (stocking density of 60 fish/m3 plus feeding diet supplemented with 3g Biogen® kg -1 diet) recorded the worst values in the leukocytes count among all experimental treatment. The promising positive results obtained in hematological blood parameters led to increase the immune status of fish and prevent mortality among all the treated fish by increasing the stoking densities of fish (Table 2). These findings were related to Biogen®, since both of allicin and ginseng (active ingredients of biogen) improved the physiological function of fish and immune response which in turn increased the ability of exposed fish to resist the stress effect of different stoking densities.
The present findings confirm those reported by Khattab et al. (2004a)they revealed that the blood hematological parameters (hemoglobin, erythrocytes count and packed cell volume percentage) in fish fed diets containing Biogen® were significantly higher than that of the control. Also, EL-Gohary et al. (2005)found the samepositive effects on blood hematological parameters of O. niloticusfed the diet supplemented withBiogen®and exposed to metrifonate (as a broad spectrum insecticide). They added that the use of Biogen® as a growth promoter in fish diet minimized the toxicity of metrifonate. Moreover,Marzouk et al. (2008a) illustrated that there were significant (P<0.05) increases in RBCs count, Hb value, PCV%, WBCs count and differential of leukocytic count in the two fish groups fed the diets supplemented with probiotics (dead Saccharomyces cerevisae yeast and both of live Bacillus subtilis and Saccharomyces cerevisae) in comparison with the control group fed on probiotic-free diet. On the other hand, Abdelhamid et al. (2002) found that Biogen® reduced blood hemoglobin of aflatoxicated O. niloticus fish.
Table (4): Effect of different stocking densities and dietary supplementation of Biogen® on blood hematological parameters of mono-sex Nile tilapia (Means ± SE)
a-b: Means in the same column different letters are significantly different (P ≤ 0.05).
Hb= Hemoglobin RBCs= Red blood cells (Erythrocytes)
PCV= Packed cell volume MCV= Mean corpuscular volume
MCH= Mean corpuscular hemoglobin MCHC= Mean corpuscular hemoglobin concentration
Platelets= Blood platelets (Thrombocytes).
Table (5): Effect of different stocking densities and dietary supplementation of Biogen® on leukocytes count and differentiation of mono-sex Nile tilapia (Means ± SE)
a-e: Means in the same column different letters are significantly different (P ≤ 0.05).
WBCs= White blood cells (Leukocytes).
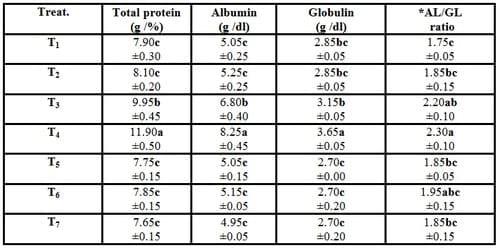
Total plasma protein, albumin, globulin and albumin /globulin ratio of the experimental fish of T4 increased significantly (P ≤ 0.05) compared with the control treatment (T1). However, increasing the stocking densities of fish (T5, T6 and T7) led to insignificant (P ≥ 0.05) decreases of total protein, albumin and globulin and to insignificant (P ≥ 0.05) increase of albumin /globulin ratio compared with the control (T1) as shown from Table (6). These findings mean that the addition of Biogen® led to significantly (P ≤ 0.05) increases of plasma proteins which indicates the improvement of the nutritional values of the diet, the growth performance, carcass composition of crude protein, physiological functions and the healthy status of the experimental fish fed on this commercial probiotic Biogen® against the high stocking density of fish. Whereas, Biogen® increases the vitality of cells by supplying oxygen to whole body, improves the immune responses and maintains the normal endocrine system (Mehrim, 2001). Moreover, Raa (1996) and Sakai (1999) revealed that some responses that are routinely reported by using immunostimulants are macrophage activation, increased phagocytosis by neutrophils and monocytes, increased lymphocyte numbers, increased serum immunoglobulins and increased lysozyme.
These results are in agreement with those obtained by Mohamed (2007) revealed the increase in plasma total protein of O. niloticus fingerlings fed on probiotic and yeast. On the other hand, Diab et al. (2002) reported that there were no significant differences in serum total protein in fish fed diets containing 0.5%, 1.0% and 1.5% of Biogen®. Moreover, Eid and Mohamed (2008)found no significant differences (P > 0.05) in plasma total protein, albumin and total globulins of fish fed the experimental diets containing different levels of probiotics (Biogen® and Pronifer®) in comparison with the control diet. Also, Wang et al. (2008b) found that there was no remarkable difference (P > 0.05) in the total serum protein, albumin and globulin concentrations and albumin/ globulin ratio between the O. niloticus supplemented with the probiotic bacterium, Enterococcus faecium ZJ4 and the control fed the basal diet.
Table (6): Effect of different stocking densities and dietary supplementation of Biogen® on plasma proteins of mono-sex Nile tilapia (Means ± SE)
a-c: Means in the same column different letters are significantly different (P ≤ 0.05).
*AL/GL ratio= Albumin /Globulin
5- Histometric examination of fish dorsal muscles
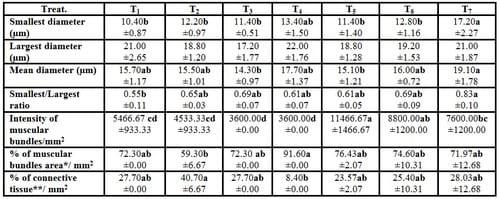
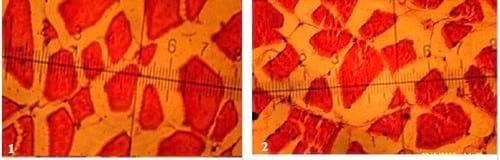
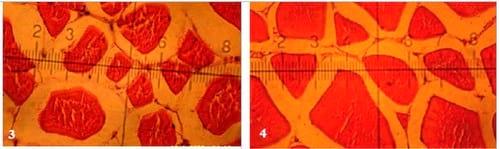
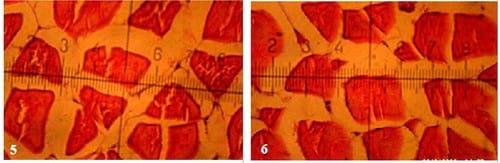
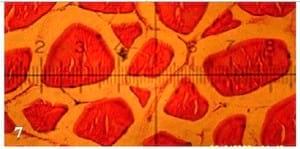
There were insignificant (P ≥ 0.05) increases of smallest diameter, mean diameter (µm), smallest/largest ratio, intensity of muscular bundles/mm2 and the percentage of muscular bundles area/mm2 of dorsal muscles of fish in T4 compared with the control(T1), but the percentageof interstitial connective tissue/mm2 decreased insignificantly (P ≥ 0.05) in T4 compared with the control. No significant differences were recorded in largest diameter (µm) among all experimental treatments. It is of interest to note that, T4 treatment realized the best growth performance and carcass composition of fish compared with the control and other treatments. On the other side, there were no adverse effects recorded on histometric measurements of fish dorsal muscles by increasing the stocking density of fish probably for the addition of the natural probiotic Biogen®. Compared with the control treatment (T1), fish in T7 reflected significantly (P ≤ 0.05) increase in smallest diameter and smallest/largest ratio, but these increases appeared not significantly (P ≥ 0.05) for mean diameter (µm), intensity of muscular bundles/mm2, the percentage of muscular bundles area (mm2) and the percentageof connective tissue (mm2) of fish dorsal muscles(Table 7 and Figs. 1-7). This means that supplementation of natural and commercial probiotic Biogen® at level of 0.3% to fish diets under high stocking densities led to improvement of most histometric characteristics of the dorsal muscles of fish compared with the control fish group (T1) which fed diet without addition of Biogen®.
These results agree with those reported by Abdelhamid et al. (2004),they found that the O. niloticus group fed diet containing 1 kg Betafin® ton -1 and 600 ml Biopolym® ton -1 was the best treatment among all treatments concerning the muscular bundles and total surface area occupied by the muscular bundles/mm2. Also, they added that the superiority of the histological structure of dorsal muscles in this treatment among all treatments was related with the high growth performance, feed and nutrients utilization and characteristics of fish production. Moreover, recently Khalil et al. (2009)revealed that mono-sex O. niloticus fed diet containing 25% replacement of fish meal by supplemented jojoba meal Simmondsia chinensis with methionine and Biogen® at level of 0.6 and 2.0 g kg -1 diet respectively (T2), led to improved significantly (P ≤ 0.05) the histometric characteristics of the dorsal muscles of mono-sex O. niloticus compared with the control treatment (T1).
Table (7): Effect of different stocking densities and dietary supplementation of Biogen® on histometric characteristics of dorsal muscles of mono-sex Nile tilapia (Means ± SE)
a-d: Means in the same row different letters are significantly different (P ≤ 0.05).
* % of muscular bundles area / mm2 = ([3.14 X (mean diameter/2)2] X Intensity of muscular bundles/mm2) X 100, whereas: the muscular bundles were considered as approximately circularity shape.
** % of connective tissue / mm2 = (1- muscular bundles area, mm2) X 100
Figs. (1 to 7): showing cross-section of muscular bundles and interstitial connective tissue of the dorsal muscles of mono-sex Nile tilapia in the 1st, 2nd, 3rd, 4th, 5th, 6th and 7th treatments respectively. (X 280 E&H stains)
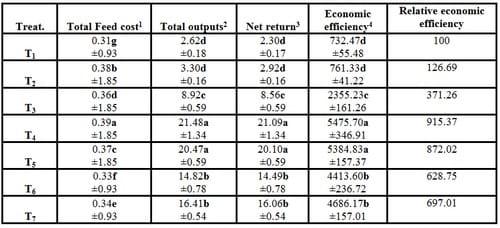
6- Economic efficiency
Results in Table (8) showed that T4 realized significant (P ≤ 0.05) increases in all economic parameters (total feed cost, total outputs, net return and economic efficiency) and high relative economic efficiency compared with the control treatment (T1) and other treatments. However, increasing stocking densities (T5, T6 and T7) led to significant (P ≤ 0.05) increases in economic parameters compared with the control (T1). From economic point of view, these positive effects due to the dietary supplementation of commercial probiotic Biogen® which alleviated the stocking density stress and significantly improved the growth performance, carcass composition, blood hematological and biochemical parameters and histometric examination of fish dorsal muscles in the present study. Additionally, Biogen® can enhance the metabolism and energy of fish body cells, raise the efficiency of feed utilization, increase the vitality of cells by supplying oxygen to whole body and improve the immune responses. Moreover, Biogen® has bactericidal effects and increases the palatability of feed, promotes the secretion of digestive fluids and stimulates the appetite (Mehrim, 2001).
Table (8): Economic efficiency (%) of different stocking densities and dietary supplementation of Biogen® of mono-sex Nile tilapia (Means ± SE)
a - g: Means in the same column different letters are significantly different (P ≤ 0.05).
1- Total feed costs (LE) = feed costs per one kg diet X feed intake
2- Total outputs (LE/Kg) = fish price X total fish production*
* Total fish production = final number of fish X fish weight gain
3- Net return per treatment (LE) = total outputs - total feed costs
4- Economic efficiency (%) = (net return/ total feed costs) X 100
The price of 1 kg ingredient used was 7.00 LE for fish meal, 2.50 LE for soybean meal, 1.50 LE for yellow corn, 1.50 LE for wheat bran, 7.50 LE for corn oil, 7.00 LE for vit. and min. premix,1.50 LE for molasses and 45.00 LE for Biogen® according to local market price at the time of study (2007) in Egypt
CONCLUSION
In conclusion, from the above mentioned results in the present study, it could be recommended the useful dietary supplementation of commercial probiotic Biogen® at a level of 0.3% with stoking density of 30 fish/m3 of mono-sex Nile tilapia fingerlings (T4).This treatment realized the best growth performance, carcass composition, blood hematological and biochemical parameters, histometric characteristics of fish dorsal muscles and economic efficiency without any adverse effects on water quality criteria. Yet, it could be required a lot of scientific efforts to maximize the commercial benefits from the environmentally-friendly commercial or natural probiotics with the local fish species.
ACKNOWLEDGEMENTS
The author would like to thank Dr. Abdelhamid M. Abdelhamid, Prof. of Animal Nutrition, Fac., of Agriculture, Mansoura Univ., Egypt for his critical reading of the manuscript and generous assistance.
REFERENCES
Abdelhamid, A.M., 1996. Field and Laboratorial Analysis in Animal Production. 1st Ed. Dar Alnashr for Universities, Cairo. (Depos. No. 11318/96) (ISBN: 977-5526-47-7).
Abdelhamid, A.M., 2000. Scientific Fundamentals of Fish Production and Husbandry. 2nd. Ed., Mansoura Faculty of Agriculture. (ISBN: 977-5526-04-1).
Abdelhamid, A.M., F.F.M. Khalil, and M.A.A. Seden, 2000. Possibility of using dried live yeast and lacto-sacc in Nile tilapia fingerlings diets. J. Agric. Sci. Mansoura Univ., 25: 4905-4911. (ISSN: 1110-0346).
Abdelhamid, A. M., F.F. Khalil, M.I. El-Barbary, V.H. Zaki, and H.S. Hussein, 2002. Feeding Nile tilpaia on Biogen® to detoxify aflatoxic diets. Proc.1st Conf. Animal and Fish Prod., Mansoura, 24&25, Sept., pp: 207-230. (ISSN: 1110-0346).
Abdelhamid, A.M., A.E., Abd El-Khalek, M.A.A. Mostafa, S.A.A. Gomaah, and F.F. Khalil, 2004. Effect of using Betafin® and/or Biopolym as natural additives in producing Nile tilapia fish in poly-culture semi-intensive system in earthen ponds. J. Agric. Sci. Mansoura Univ., 29: 3149-3162. (ISSN: 1110-0346).
Abdelhamid, M.A., M.A. Ibrahim, N.A. Maghraby and A.A.A. Soliman, 2007. Effect of dietary supplemented of betaine and/or stocking density on performance of Nile tilapia. J. Agric. Sci. Mansoura Univ., 32: 167-179. (ISSN: 1110-0346).
Abd El- Hakim, N.F., M.N. Bakeer and M.A. Soltan, 2002. Water Environment for Fish Culture. Deposition No.: 4774, (ISBN: 977-298-228-5).
AOAC., 2000. Association of Official Analytical Chemists of official methods of analysis, 17th Ed. Washington, DC. (http://www.covance.com/analytical/svc_nutrients_min.php).
Bakeer, M.N., M.A.A. Mostafa and A.Z. Higaze, 2007. Effect of fish size and density at initial stocking on growth performance and fish marketable size. J. Agric. Sci. Mansoura Univ., 32: 1803-1813. (ISSN: 1110-0346).
Diab, A.S., G.O. El-Nagar, and Y.M. Abd-El-Hady, 2002. Evaluation of Nigella sativa L. (Black seeds; Baraka), Allium sativum (garlic) and Biogen® as a feed additives on growth performance and immuostimulants of Oreochromis niloticus fingerlings. Suez Canal Vet. Med. J., 2002, 745-775. (ISSN: 1110-6298).
Diana, J.S., Y. Yi and C.K. Lin, 2004. Stoking densities and fertilization regimes for Nile tilapia (Oreochromis niloticus) production in ponds with supplemental feeding. Proceedings of 6th International Symposium on Tilapia in Aquaculture, Roxas Boulevard, Manila, Philippines, pp. 487-499. (http://ag.arizona.edu/azaqua/ista/ista6/Abstracts6.htm).
Doumas, B.T. and H.G. Biggs, 1972. Determination of serum albumin. In: Standard Method of Clinical Chemistry.Vol.7 Edited by G.R. Cooper, New York Academic press.
Duncan, D.B., 1955. Multiple range and multiple F-test. Biometrics, 11:1-42. (http://scholar.google.com.br/scholar?q=Multiple%20range%20and%20multiple%20F%20tests).
Eid, A. and K.A. Mohamed, 2008. Effect of using probiotic as growth promoter in commercial diets for monosex Nile tilapia (Oreochromis niloticus) fingerlings. 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt, 12-14 Oct., pp: 241-253. (ISBN: 978-1-888807-18-9).
EL-Gohary, M.S., S.G. Mohamed, R.H. Khalil, S. EL-Banna and M.K. Soliman, 2005. Immunosuppressive effects of metrifonate on Oreochromis niloticus. Egyptian Journal of Aquatic Research, 31: 448-458. (ISSN: 1110-0354). (http://hdl.handle.net/1834/1218).
EL-Haroun, E.R., 2007. Improved growth rate and feed utilization in farmed African catfish Clarias gariepinus (Burchell 1822) thought a growth promoter Biogen® supplementation. Journal of Fisheries and Aquatic Science, 2: 319-327. (ISSN: 1816-4927). (DOI: 10.3923/jfas.2007.319.327).
EL-Haroun, E.R., A. MA-S Goda, and M.A. Kabir Chowdhury, 2006. Effect of dietary probiotic Biogen® supplementation as a growth promoter on growth performance and feed utilization of Nile tilapia Oreochromis niloticus (L.). Aquaculture Research, 37: 1473-1480. (http://dx.doi.org/10.1111/j.1365-2109.2006.01584.x) (DOI: 10.1111/j.1365-2109.2006.01584.x).
Ellis, T., B. North, A.P. Scott, N.R. Bromage, M. Porter and D. Gadd, 2002. The relationships between stocking density and welfare in farmed rainbow trout. J. Fish Biol., 61: 493-531. (DOI: 10.1111/j.1095-8649.2002.tb00893.x).
El-Sayed, A.M., 2002. Effect of stocking density and feeding levels on growth and feed efficiency of Nile tilapia Oreochromis niloticus L. fry. Aquaculture Research, 33: 621-626. (http://dx.doi.org/10.1046/j.1365-2109.2002.00700.x). (DOI: 10.1046/j.1365-2109.2002.00700.x).
Essa, M.A. and M.E. Salama, 1994 Salinity tolerance and reproductive performance of Nile tilapia, Oreochromis niloticus. Delta Journal Science, 18: 239-261. (ISSN: 1012-5965).
Fitzsimmons, K., 2000. Tilapia: the most important aquaculture species of the 21st century. In: Fitzsimmons, K. and Filho, J. C. (eds), Proceedings from the 5th International Symposium on Tilapia Aquaculture, Vol. 1. Rio de Janeiro, Brazil, pp. 3-8. (http://ag.arizona.edu/azaqua/ista/order.html).
Fitzsimmons, K., 2004. Development of new products and markets for the global tilapia trade. In: Proceeding of the 6th International Symposium on Tilapia in Aquaculture, Manila, Philippines (ed. by R. Bolivar, G. Mair & K. Fitzsimmons), pp.624-633. BFAR, Philippines. (http://ag.arizona.edu/azaqua/ista/ista6/Abstracts6.htm).
Fuller, R., 1992. Probiotics. The Scientific Basis. Ed. R. Fuller. Chapman & Hall. London, New York, Tokyo, Melbourne, Madras. (ISBN-13: 978-0412736100).
Gall, G.A.E. and Y. Bakar, 1999. Stocking density and tank size in the design of breed improvement programs for body size of tilapia. Aquaculture, 173:197-205. (DOI: 10.1016/S0044-8486(98)00487-6).
Gatesoupe, F.J., 2002. Probiotic and formaldehyde treatments of Artemia nauplii as food for larval pollack, Pollachius pollachius. Aquaculture, 212: 347-360. (http://www.ifremer.fr/docelec/notice/2002/notice393-EN.htm). (DOI:10.1016/S0044-8486(02)00138-2).
Gornall, A.G., G.J. Bardawill and M.M. Parid, 1949. Method of determination protein in serum blood .J. Biol. Chem., 177: 751.
Khalil, F.F., F.H. Farrag and A.I. Mehrim, 2009. Evaluation of using Jojoba meal Simmondsia chinensis supplemented with methionine and Biogen® instead of fish meal in diet for mono-sex Nile tilapia Oreochromis niloticus. Egyptian J. Nutrition and Feeds, 12 (1): 141-156. (ISSN: 1110-6360).
Khattab, Y.A.E., A.M.E. Shalaby, S.M. Sharaf, H.I. El-Marakby and E.H. Rizkalla, 2004a. The physiological changes and growth performance of the Nile tilapia Oreochromis niloticus after feeding with Biogen® as growth promoter. Egypt, J. Aquat. Bio. and Fish., 8: 145-158. (ISSN: 1110-6131).
Khattab, Y.A.E., A. Mohsen and M.H. Ahmed, 2004b. Effect of protein level and stocking density on growth performance, survival rate, feed utilization and body composition of Nile tilapia fry (Oreochromis niloticus L.). Proceedings of 6th International Symposium on Tilapia in Aquaculture, Roxas Boulevard, Manila, Philippines, pp. 264-276. (http://ag.arizona.edu/azaqua/ista/ista6/Abstracts6.htm).
Knud-Hansen, C.F. and T.R. Batterson, 1994. Effect of fertilization frequency on the production of Nile tilapia
Oreochromis niloticus. Aquaculture, 123: 271-280. (
ISSN: 0044-8486). (

DOI:10.1016/0044-8486(94)90065-5).
Lara-Flores, M., M.A. Olvera-Novoa, B.E. Guzmán-Méndez and W. López-Madrid, 2003. Use of the bacteria Streptococcus faecium and Lactobacillus acidophilus, and the yeast Saccharomyces cerevisiae as growth promoters in Nile tilapia Oreochromis niloticus. Aquaculture, 216: 193-201. (http://dx.doi.org/10.1016/S0044-8486(02)00277-6). (DOI: 10.1016/S0044-8486 (02)00277-6).
Liu, K.M. and W.B. Chang, 1992. Bioenergetic modeling of effect of fertilization, stocking density, and spawning on growth of the Nile tilapia, Oreochromis niloticus (L.). Aquaculture and Fisheries Management, 23: 291-301. (DOI: 10.1111/j.1365-2109.1992.tb00772.x).
Macdonald, P., R.A. Edwards and J.F.D. Greenhalgh, 1973. Animal Nutrition, 2nd Ed., Longman, London.
Marzouk, M.S., M.M. Moustafa and N.M. Mohamed, 2008a. Evaluation of immunomodulatory effects of some probiotics on cultured Oreochromis niloticus. Proceedings of 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt, pp. 1043-1058. (ISBN: 978-1-888807-18-9).
Marzouk, M.S., M.M. Moustafa and N.M. Mohamed, 2008b. The influence of some probiotics on the growth performance and intestinal microbial flora of Oreochromis niloticus. Proceedings of 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt, pp. 1059-1071. (ISBN: 978-1-888807-18-9).
Mehrim, A.I.M. (2001). Effect of some chemical pollutant on growth performance, feed and nutrient utilization of tilapia. M.Sc. Thesis, Saba- Basha Fac. of Agric., Alex. Univ.
Mohamed, K.A., 2007. Effect of using probiotic and yeast as growth promoters in commercial diet of tilapia (Oreochromis niloticus) fingerlings. Agricultural Research Journal, Suez Canal University, 7: 41-47. (ISSN: 1110-6999).
Mohamed, K.A., B. Abdel Fattah and A.M.S. Eid, 2007. Evaluation of using some feed additives on growth performance and feed utilization of monosex Nile tilapia (Oreochromis niloticus) fingerlings. Agricultural Research Journal, Suez Canal University, 7: 49-54. (ISSN: 1110-6999).
Mombelli, B. and M.R. Gismondo, 2000. The use of probiotics in medicinal practice. International Journal of Antimicrobial Agents, 16: 531-536. (

DOI:10.1016/S0924-8579(00)00322-8).
Ouwehand, A.C., S. Salminen and E. Isolauri, 2002. Probiotics: an overview of beneficial effects. Antonie van Leewenhoek, 82: 279-289.http://www.ingentaconnect.com/klu/anto/2002/00000082/F0040001/05098352).
Pearse, G.W., 1968. Histological effects and diagnostic problems of mycotoxins in poultry. Proc. 25th West States Poult. Dis. Conf., pp.76-79.
Raa, J., 1996. The use of immunostimulatory substances in fish and shellfish farming. Reviews in Fisheries Science, 4: 229-288. (http://w3.dsi.uanl.mx/publicaciones/maricultura/acuiculturaV/raa.pdf).
Rakocy, J.E., D.S. Bailey, E.S. Thoman and R.C. Shultz, 2004. Intensive tank culture of tilapia with a suspended, bacterial-based, treatment process. Proceedings of 6th International Symposium on Tilapia in Aquaculture, Roxas Boulevard, Manila, Philippines, pp. 584-598. (http://ag.arizona.edu/azaqua/ista/ista6/Abstracts6.htm).
Sakai, M., 1999. Current research status of fish immunostimulants. Aquaculture, 172: 63-92. (DOI: 10.1016/S0044-8486(98)00436-0).
SAS, 1997. SAS/STAT Guide for personal computer. SAS Inst. Cary, N. C. (www.informatik.uni-trier.de/~ley/db/conf/sas/sas98.html - 15k), (ISBN: 3-540-65014-8).
Senok, A.C., A.Y. Ismaeel and G.A. Botta, 2005. Probiotics: facts and myths. Clinical Microbiology and Infection, 11: 958-966. (DOI: 10.1111/j.1469-0691.2005.01228.x).
Srour, T.M., 2004. Effect of ochratoxin-A with or without Biogen® on growth performance, feed utilization and carcass composition of Nile tilapia (Oreochromis niloticus) fingerlings. J. Agric. Sci. Mansoura Univ., 29: 51-61. (ISSN: 1110-0346). (http://app2.mans.edu.eg/eulc/Libraries/StaffPapers/StaffPaper.aspx?fn=BrowseByAuthor&ScopeID=1.1.&AuthorName=Srour%2c+T.+M.).
Sullivan, A. and C.E. Nord, 2002. The place of probiotics in human intestinal infections. International Journal of Antimicrobial Agents, 20: 313-319. (DOI: 10.1016/S0924-8579(02)00199-1).
Tacon, A.G.J., 1990. Standard Methods for the Nutrition and Feeding of Farmed Fish and Shrimp, Vol. II & III. Argent Laboratories Press, Washington, USA.
Vine, N.G., W.D. Leukes, and H. Kaiser, 2006. Probiotics in marine larviculture. FEMS Microbiol Rev., 30: 404-427. (http://www.ncbi.nlm.nih.gov/pubmed/16594964). (ISSN: 0168-6445 ).
Vine, N.G., W.D. Leukes, H. Kaiser, J. Baxter and T. Hecht, 2004. Competition for attachment of aquaculture candidate probiotic and pathogenic bacteria on fish intestinal mucus. J. Fish Dis., 27: 319-326. (http://dx.doi.org/10.1111/j.1365-2761.2004.00542.x). (DOI:10.1111/j.1365-2761.2004.00542.x).
Wang, Y.B., J. Li and J. Lin, 2008a. Probiotics in aquaculture: Challenges and outlook. Aquaculture, 281: 1-4. (DOI:10.1016/j.aquaculture.2008.06.002 ).
Wang, Y.B., Z. Tian, J. Yao, and W. Li, 2008b. Effect of probiotics, Enteroccus faecium, on tilapia (Oreochromis niloticus) growth performance and immune response. Aquaculture, 277: 203-207. (DOI:10.1016/j.aquaculture.2008.03.007).
Weichsebum, T.E., 1946. Method for determination of albumin in serum blood. Amer. J. Clin. Pathol., 16-40.
Yi, Y., K. Fitzsimmons, W. Saelee and C. Potjanee, 2004. Stocking densities of Nile tilapia in shrimp ponds under different feeding strategies. Proceedings of 6th International Symposium on Tilapia in Aquaculture, Roxas Boulevard, Manila, Philippines, pp. 402-420. (http://ag.arizona.edu/azaqua/ista/ista6/Abstracts6.htm).




.jpg&w=3840&q=75)


.jpg&w=3840&q=75)