Growing incidence of Piscirickettsia infection in fish worldwide: mechanisms for prevention and control
The presence and importance of Piscirickettsia-like bacteria in mammals have been long recognized, but only in recent years could they be identified and characterized in aquatic animals. For this reason, it was not until the late 1980s that Rickettsia agents were linked with major diseases in fish, and subsequently attributed as the cause of substantial economic losses due to disease-related mortality in the 1990s.
Piscirickettsiosis occurrence
Piscirickettsia salmonis was the first ‘rickettsia-like’ bacterium identified as a pathogenic agent in fish. Since the first reports of piscirickettsiosis emerged from Chile in the late 1980s (Bravo, 1989), Piscirickettsia-like bacteria have been identified with increasing frequency in a variety of fish species, from both fresh and salt water environments around the world (Table 1).
Although the first reported cases of Piscirickettsia were in salmonids (Fryer and Mauel, 1997), Piscirickettsia-like bacteria are now linked with disease syndromes in non-salmonid fish.
Mortalities have occurred in white sea bass (Atractoscion nobilis) (Chen et al., 1999), black sea bass (Dicentrarchus spp.) (Comps et al., 1996), tilapia (Oreochromis, Tilapia and Sarotherodon spp.) (Chen et al., 1994; Wada et al., 1995) and blue-eyed plecostomus (Panaque suttoni) (Khoo et al., 1995).
Piscirickettsiosis and piscirickettsiosis-like diseases have affected aquaculture productivity, profitability, species compatibility with commercial rearing, and fish transport.
Piscirickettsiosis and syndromes caused by similar bacteria are an emerging disease complex that could increasingly limit fish production (Mauel and Miller, 2002).
In 1995, Cvitanich et al. described a ‘rickettsia-like organism’ (RLO), U2, in Atlantic salmon reared in sea water and estuaries and propagated in cellular cultures. U2-related mortality rates are variable, but because of its therapeutic response, they remain relatively minor compared with those from salmonid rickettsial septicemia (SRS). In 1997, RLO agents were observed in Turbot (Scophthalmus maximus) reared in northwest Chile, nevertheless they did not generate losses, since the mortalities were only linked with a specific vitamin deficiency.
Table 1. Piscirickettsia-like organisms in fish from 1939 to 2001.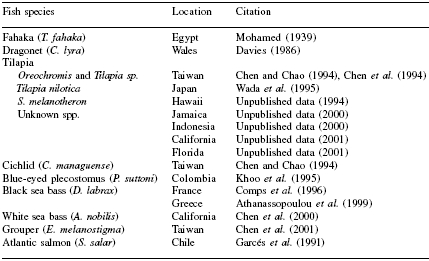
Mauel and Miller (2002)
Soon after the presence, characterization, and diverse epidemiologic aspects of this agent in Chile were published, several other publications arose indicating that an agent with similar characteristics was present in reared salmon in Norway (Olsen et al., 1993; Olsen et al., 1997), Scotland (Reid et al., 2004), Ireland (Palmer et al., 1997) and Canada (Cusack et al., 1997; Jones et al., 1998).
In all of these countries except Norway, reports of piscirickettsiosis have been increasing for the last 5–10 years with farmers experiencing related economic losses. In Europe and California, piscirickettsia-like organisms (PLOs) have been observed in and isolated from sea bass species. These epizootic diseases have caused high rates of mortality.
In Taiwan and Hawaii, PLOs have wreaked havoc on tilapia farms; 37 Taiwanese farms suffered mortalities up to 95%, and in Hawaii, the transport of fish between Oahu and other Hawaiian islands is now restricted with some farmers questioning the feasibility of farming tilapia (Mauel and Miller, 2002).
At present, PLO infections have been found in tilapia in Central America, striped bass/tilapia in California, and cod in Norway.
Prevention and control strategies
TRADITIONAL STRATEGIES
In the Chilean salmon industry, losses due to mortalities attributed to SRS exceed $100 million annually, and today it represents the most significant disease threat relevant to brackish and sea water aquaculture. Vaccines developed since 2000 have not been sufficiently effective, however newer versions of these vaccines are showing small but gradual improvement. During the final third of rearing in sea water before harvest, when coincidental outbreaks are more critical and complex to control, biomass losses are potentially the greatest. At this stage of production, existence of an effective vaccine against SRS would be of great value.
Concomitantly, the effectiveness of oral antibiotic treatment in the field has been inconsistent and declining, and consequently, overall mortalities in salmonid facilities continue to increase. Antibiotics have been losing efficiency for varied reasons, especially bacterial resistance. Moreover, blood absorption rates are close to or at the lower effective limit for effectiveness in the majority of fish species. For the rest, it is in those months before harvest when the use of antibiotics is not possible, given the need to eliminate the risk of chemical residues, in which fish are the most vulnerable to disease. Animal health in these months is critical in determining aquaculture profitability.
Compared with SRS, antibiotics work better in combating PLO infections. PLO vaccines also seem to be generating better results, although further research is needed. An understanding of the antigenic characteristics of PLOs and the pathology of the disease is vital to help develop prevention and control strategies.
ALTERNATIVE STRATEGIES
In summary, P. salmonis was the first RLO recognized as a pathogen in fish. Since its identification a decade ago, PLOs have been identified with increasing frequency at numerous locations worldwide in a variety of fish species in both fresh (Bravo, 1994) and salt water. Many of these agents have been identified as P. salmonis by serological and DNA sequencing methods, but the relatedness of many others to P. salmonis remains unknown. Epizootic diseases caused by these agents often result in high mortalities and great economic losses (Mauel and Miller, 2002).
To develop successful prevention and control strategies it is vital that strategies be diverse and developed from a holistic perspective, including the use of appropriate handling measures, suitable biosafety standards, effective vaccines, and of course the application of immunostimulants and immune modulators.
In Chile at least, the application of alternative strategies to combat PLOs is beginning to have a significant effect, especially since traditional therapies have proven relatively ineffective. There is a pressing need to reduce the use of antibiotics, handling measures are only partially effective, and vaccines to date are not highly effective.
Research trials and field trials are needed to objectively demonstrate whether immunostimulants and immune modulators are cost effective. Such demonstrations are currently very much needed to reduce or eliminate the uncertainty that exists about the use of these natural substances to combat disease in fish.
Certainly, the difficulties in conducting precise scientific studies in controlled or semi-controlled tanks in fresh water and sea water environments are minor compared with the difficulties in conducting precise experiments in sea cages. Often experimental results are not sufficiently consistent to satisfy fish farmers, and do not sufficiently convey economic benefits.
For this reason, it is important that research and field trials be carefully designed, statistically rigorous, and well executed. Nevertheless, few supervised trials have been conducted and often those that have been were performed ‘in-house’ by companies so that objectivity remains in question. Thus, there is an urgent need to rigorously demonstrate the efficiency and cost benefit of using immunostimulants and immune-modulators in combating PLOs in fish by using well designed field trials.
References
Author: PATRICIO BUSTOSF. Athanassopoulou, O. Sabatakou, D. Groman and T. Prapas. 1999. First incidence of rickettsia-like infections in cultured sea bass (Dicentrarchus labrax L.) in Greece. In: Proceedings of the Ninth International Conference, European Association of Fish Pathologist. Rhodes, Greece, September 19-14 (Poster Abstract).
Bravo, S. and M. Campos. 1989. Síndrome del Salmón Coho. Chile Pesquero 54:47- 48.
Bravo, S. 1994. Piscirickettsiosis in freshwater. Bull. Eur. Assoc. Fish. Pathol. 14:137- 138.
Chen, S.C., M.C. Tung, S.P. Chen, J.F. Tsai, P.C. Wang, R.S. Chen, S.C. Lin and A. Adams. 1994. Systemic granulomas caused by a rickettsia-like organism in Nile tilapia, Oreochromis niloticus (L.), from southern Taiwan. J. Fish Dis. 17:591-599.
Chen, M.F., J.A. Apperson, T. Gunther, M.L. House, D.B. Antonio, K.B. Andree, S.Z. Yun, M.A. Adkison, G.D. Marty and R.P. Hedrick. 1999. Isolation and partial characterization of a rickettsia associated with large scale mortality of cultured white sea bass (Atractoscion nobilis) in California. In: Proceedings of the AFS/Fish Health Section 1999 Annual Meeting and Western Fish Disease Workshop. Twin Falls, Idaho, June 9-11 (Abstract).
Chen, S.C., P.C. Wang, M.C. Tung, K.D. Thompson and A. Adams. 2000. A Piscirickettsia salmonis-like organism in grouper, Epinephelus melanostigma, in Taiwan. J. Fish Dis. 23:415-418.
Chen, R.S. and C.B. Chao. 1994. Outbreaks of a disease caused by a rickettsia-like organism in cultured tilapias in Taiwan. Fish Pathol. 29:61-71.
Comps, M., J.C. Raymond and G.N. Plassiart. 1996. Rickettsia-like organism infecting juvenile sea-bass Dicentrarchus labrax. Bull. Eur. Assoc. Fish. Pathol. 16:30-33.
Cusack, R., D. Groman and S. Jones, S., 1997. The first reported rickettsial infections of Atlantic salmon in eastern North America. In: Proceedings of the European Association of Fish Pathologists VIII International Conference on Diseases of Fish and Shellfish. Edinburgh, Scotland, September 14-19 (Abstract).
Cvitanich J., O. Gárate and C.E. Smith. 1995. Isolation of a new rickettsia-like organism from Atlantic salmon in Chile. FHS/AFS Newsletter 23:1-2.
Davies, A.J. 1986. A rickettsia-like organism from dragonets, Callionymus lyra L. (Teleostei: Callionymidae) in Wales. Bull. Eur. Assoc. Fish Pathol. 6:103.
Fryer, J.L. and M.J. Mauel. 1997. The rickettsia: an emerging group of pathogens in fish. Emerg. Inf. Dis. 3:137-144.
Garcés, L.H., J.J. Larenas, P.A. Smith, S. Sandino, C.N. Lannan and J.L. Fryer. 1991. Infectivity of a rickettsial isolated from coho salmon (Oncorhynchus kisutch). Dis. Aquat. Org. 11:93-97.
Jones, S.R.M., R.J.F. Markham, D.B. Groman and R.R. Cusack. 1998. Virulence and antigenic characteristics of a cultured Rickettsia-like organism isolated from farmed Atlantic salmon (Salmo salar) in eastern Canada. Dis. Aquat. Org. 33:25-31.
Khoo, L., P.M. Dennis and G.A. Lewbart. 1995. Rickettsia-like organisms in the blueeyed plecostomus, Panaque suttoni (Eigenmann and Eigenmann). J. Fish Dis. 18:157- 164.
Mauel M.J. and D.L. Miller. 2002. Piscirickettsiosis and piscirickettsiosis-like infections in fish: a review. Vet. Microbiol. 87(4):279-89.
Mohamed, Z. 1939. The discovery of a rickettsia in a fish. Minist. Agric. Cairo, Tech. Sci. Serv., Vet. Sect. Bull. 214, p. 6.
Olsen, A.B., O. Evensen, L. Speilberg, H.P. Melby and T. Hastein. 1993. Ny laksesykdom forarsaket av rickettsie. Norsk Fiskeoppdrett NR. 12:40–41.
Olsen, A.B., H.P. Melby, L. Speilberg, O. Evensen and T. Hastein. 1997. Piscirickettsia salmonis infection in Atlantic salmon Salmo salar in Norway-epidemiological, pathological and microbiological findings. Dis. Aquat. Org. 31:35-48.
Palmer, R., M. Ruttledge, K. Callanan and E. Drinan. 1997. A Piscirickettsiosis-like disease in farmed Atlantic salmon in Ireland – isolation of the agent. Bull. Eur. Assoc. Fish Pathol. 17:68–72.
Reid, H.I., A.A. Griffen and T.H. Birkbeck. 2004. Isolates of Piscirickettsia salmonis from Scotland and Ireland show evidence of clonal diversity. Appl Environ Microbiol. 70(7):4393–4397.
Wada, S., K. Hatai and T. Shiomitsu. 1995. A disease with liver swelling and nodule formation in cultured tilapia. In: Proceedings of the 1995 Spring Meeting Japan. Soc. Fish Pathol., Tokyo, Japan, March 30–31, 1995 (Abstract in Japanese).
ADL Diagnostic Chile Ltda. (Aquatic Diseases Laboratory), Castro, Chiloé Island, Chile


